Efficacy of Rikkunshito on Chemotherapy-Induced Nausea and Vomiting in Patients With Uterine Corpus or Cervical Cancer Treated With Cisplatin-Based Regimen-Placebo-controlled, Double-Blind, Randomized Confirmatory Study (JORTC-KMP03)
- PMID: 40156342
- PMCID: PMC11954530
- DOI: 10.1177/15347354251329346
Efficacy of Rikkunshito on Chemotherapy-Induced Nausea and Vomiting in Patients With Uterine Corpus or Cervical Cancer Treated With Cisplatin-Based Regimen-Placebo-controlled, Double-Blind, Randomized Confirmatory Study (JORTC-KMP03)
Abstract
Background: The current standard treatment for chemotherapy-induced nausea and vomiting (CINV) with standard antiemetics is insufficient. Rikkunshito, a Japanese traditional herbal medicine, has been shown to improve cisplatin-induced anorexia and functional dyspepsia, and our exploratory study found that rikkunshito has an additive beneficial effect on CINV in patients with uterine corpus and cervical cancer receiving cisplatin containing chemotherapy (JORTC KMP-02).
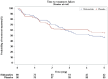
Methods: One hundred eighty patients with uterine corpus or cervical cancer who were scheduled to receive treatment with a cisplatin based regimen as initial chemotherapy were enrolled across 17 Japanese institutions. Patients were randomized with a 1:1 equal allocation ratio to the rikkunshito group or placebo groups and given oral administration on days 1 to 5 with standard antiemetics (granisetron, aprepitant, and dexamethasone). The primary endpoint was complete response (CR; no vomiting or rescue medication) during the delayed phase (24-120 hours after cisplatin treatment). The secondary endpoints were complete control (CC; CR without significant nausea) and total control (TC; CR without nausea) rates during the overall (0-120 hours), acute (0-24 hours), and delayed phases, as well as the CR rate during the overall and acute phases, time to treatment failure, degree of nausea and appetite during the overall phase, and adherence to the intervention.
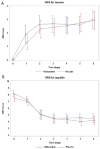
Results: The CR rate in the delayed phase was similar between the rikkunshito group and control groups (50.6% vs 58.9%, P = .2631), as were the secondary endpoints: CR rates in the overall and acute phases, CC and TC rates in the overall, acute, and delayed phases, degrees of nausea and appetite, and time to treatment failure.
Conclusion: Rikkunshito had no additive effect on CINV prevention in patients with uterine corpus or cervical cancer who were treated with a cisplatin based regimen and standard antiemetics.
Clinical trial registration: https://jrct.mhlw.go.jp/re/reports/detail/66957, identifier jRCT1011190007.
Keywords: Rikkunshito; cervical cancer; chemotherapy-induced nausea and vomiting; cisplatin; herbal medicine; uterine corpus cancer.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The study drug was provided by Tsumura & Co. The company plays no role in the study’s design, data collection and analysis, publication decision, or preparation of the manuscript. SOh received a speaker fee from Tsumura & Co. for 36 months before publication. The other authors state that the study was conducted without any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical