Prognostic Significance of Chaperonin-Containing Tailless Complex Polypeptide 6A (CCT6A) in Ewing Sarcoma
- PMID: 40156427
- PMCID: PMC12174516
- DOI: 10.31557/APJCP.2025.26.3.1079
Prognostic Significance of Chaperonin-Containing Tailless Complex Polypeptide 6A (CCT6A) in Ewing Sarcoma
Abstract
Background: Chaperonin-Containing Tailless complex polypeptide 6A (CCT6A) is considered one of the biomarkers that play a role in cancer initiation, progression and resistance to body defenses and to anti-cancer drugs. Its exact prognostic role in Ewing sarcoma (ES) remains uncertain. The aim of this study is to assess immunohistochemical (IHC) expression of CCT6A in ES, and to assess the relation of its expression to different clinicopathological parameters and evaluate its prognostic significance in ES cases.
Methods: This retrospective cohort study included 35 cases diagnosed as ES at the Oncology Center of Mansoura University (OCMU), Faculty of Medicine, Egypt. Clinicopathological and survival data were collected. IHC for CCT6A was performed and correlated with clinicopathological parameters and patients' prognosis.
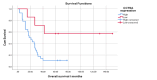
Results: High IHC expression of CCT6A was found in 28 cases out of the studied 35 ES cases (80%), while 7 cases only (20%) showed low CCT6A expression. High CCT6A expression showed significant association with tumor size ≥8 cm (P= 0.01), treatment with adjuvant radiotherapy either for local control, infiltrated surgical margins or poor histopathological response to chemotherapy (P= 0.01), poor histopathological response to chemotherapy (P= 0.02), and HUVOS grades I and II (P= 0.01). High CCT6A expression was found to be a predictor of shorter overall survival and was an independent poor predictive factor by multivariate analysis (P= 0.03).
Conclusion: High CCT6A expression in ES associates with large tumor size, treatment with adjuvant radiotherapy for aforementioned indications, poor histopathological response to chemotherapy, and HUVOS grades I and II. Its high expression also independently predicts poor overall survival in ES patients. It may be used as a useful biomarker to predict prognosis of ES.
Keywords: CCT6A; Ewing sarcoma; Prognosis; survival.
Conflict of interest statement
The authors declare no relevant financial affiliations or conflicts of interest.
Figures


Similar articles
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
An immune-related gene signature for determining Ewing sarcoma prognosis based on machine learning.J Cancer Res Clin Oncol. 2021 Jan;147(1):153-165. doi: 10.1007/s00432-020-03396-3. Epub 2020 Sep 23. J Cancer Res Clin Oncol. 2021. PMID: 32968877 Free PMC article.
-
CCT6A, a novel prognostic biomarker for Ewing sarcoma.Medicine (Baltimore). 2021 Jan 29;100(4):e24484. doi: 10.1097/MD.0000000000024484. Medicine (Baltimore). 2021. PMID: 33530265 Free PMC article.
-
High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with primary metastatic Ewing sarcoma.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD011405. doi: 10.1002/14651858.CD011405.pub2. Cochrane Database Syst Rev. 2021. PMID: 34472082 Free PMC article.
References
-
- Wei S, Siegal GP. Small round cell tumors of soft tissue and bone. Arch Pathol Lab Med. 2022;146(1):47–59. - PubMed
-
- Machado I, Yoshida A, López-Guerrero JA, Nieto MG, Navarro S, Picci P, Llombart-Bosch A. Immunohistochemical analysis of NKX2 2, ETV4, and BCOR in a large series of genetically confirmed Ewing sarcoma family of tumors. Pathol Res Pract. 2017;213(9):1048–53. - PubMed
-
- El Nadi E, El Ghoneimy A, El Shafiey M, Taha H, Zaghlool MS, Zaki I, et al. Ewing Sarcoma/Peripheral Neuroectodermal Tumor of Bone and Soft Tissue in Infants Egypt: Children Cancer Hospital of Egypt. Cancer Res J. 2021;9(1)
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

