On subcellular distribution of the zinc finger 469 protein (ZNF469) and observed discrepancy in the localization of endogenous and overexpressed ZNF469
- PMID: 40156465
- PMCID: PMC12226416
- DOI: 10.1002/2211-5463.70034
On subcellular distribution of the zinc finger 469 protein (ZNF469) and observed discrepancy in the localization of endogenous and overexpressed ZNF469
Abstract
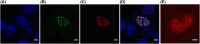
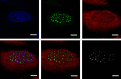
The zinc finger 469 gene (ZNF469) is a single-exon gene predicted to encode a protein of 3953 amino acids. Despite pathogenic ZNF469 variants being associated with Brittle Cornea Syndrome (BCS), relatively little is known about ZNF469 beyond its participation in regulating the expression of genes encoding extracellular matrix proteins. In this study, we examined the expression and intracellular localization of ZNF469 in different cell lines. The level of ZNF469 mRNA varied from low levels in HEK293 cells to high levels in HeLa cells and primary fibroblasts. Antibodies against ZNF469 reacted among others with a protein of approximately 400 kDa in immunoblot analysis, which was mainly present in the insoluble fraction of the cytoplasm. Immunofluorescence analysis of interphase cells showed small cytoplasmic puncta and weak nuclear staining. In dividing HeLa cells, the antibodies recognized foci that also stained for proteasomes. In transfected cells, ZNF469 was observed mainly in foci resembling nuclear speckles in interphase and at the midbody during mitosis. The nuclear foci showed overlapping staining with proteasomes. In live cell imaging, liquid-like properties of the nuclear foci were recorded as they changed shape and position and occasionally fused with each other. During stress granule formation, cytoplasmic foci showed overlapping staining with G3BP1. Finally, in silico analysis revealed large intrinsically disordered regions with multiple low complexity domains in ZNF469. Our data indicate that ZNF469 forms aggregates possibly as biomolecular condensates when overexpressed. However, care must be taken when analyzing the intracellular distribution of ZNF469 due to the discrepancy in the localization of endogenous ZNF469 and overexpressed ZNF469 in transfected cells.
Keywords: biomolecular condensates; intrinsically disordered regions; midbody; nuclear speckles; proteasomes; stress granules.
© 2025 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
A.E.C. Mellgren, None; I. Cristea, None; T. Stevenson, None; E. Spriet, None; P.M. Knappskog, None; S.O. Bøe, None; H. Kranz, None; S.N. Grellscheid, None; E. Rødahl, None.
Figures




References
-
- Rohrbach M, Spencer HL, Porter LF, Burkitt‐Wright EM, Bürer C, Janecke A, Bakshi M, Sillence D, Al‐Hussain H, Baumgartner M et al. (2013) ZNF469 frequently mutated in the brittle cornea syndrome (BCS) is a single exon gene possibly regulating the expression of several extracellular matrix components. Mol Genet Metab 109, 289–295. - PMC - PubMed
-
- Christensen AE, Knappskog PM, Midtbø M, Gjesdal CG, Mengel‐From J, Morling N, Rødahl E and Boman H (2010) Brittle cornea syndrome associated with a missense mutation in the zinc‐finger 469 gene. Invest Ophthalmol Vis Sci 51, 47–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

