Systematic Literature Review and Network Meta-Analysis of Clinical Efficacy and Safety of Topical Treatments for Patients with Atopic Dermatitis
- PMID: 40156696
- PMCID: PMC12033160
- DOI: 10.1007/s13555-025-01390-6
Systematic Literature Review and Network Meta-Analysis of Clinical Efficacy and Safety of Topical Treatments for Patients with Atopic Dermatitis
Abstract
Introduction: In Japan, atopic dermatitis (AD) is one of the most common skin diseases, with the number of patients steadily increasing in recent years. Thus, it is crucial to assess the efficacy and safety of currently existing and recently introduced new treatments.
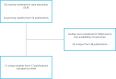
Methods: A systematic literature review (SLR) and network meta-analysis (NMA) was conducted to evaluate the clinical efficacy and safety of existing standard topical therapies and new topical treatments for AD. Medline, Embase, Cochrane, and ICHUSHI were used to select studies. The Eczema Area and Severity Index (EASI) score and Investigator Global Assessment (IGA) score were efficacy outcomes, whereas any serious adverse events (AEs), acne, and skin infections were safety outcomes. A Bayesian multiple treatment NMA with fixed effects was performed. Odds ratio with 95% credible interval (CrI) was used to compare the outcomes of different topical medications including placebo for AD.
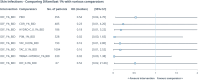
Results: A total of 11 randomised controlled trials (RCTs) conducted in adult patients with varying degrees of AD severity were selected for NMA. The systematic review showed improvement in EASI scores with difamilast 0.3% and 1% and tacrolimus 0.1% as well as in IGA score success rates with difamilast 1%, delgocitinib 3%, and tacrolimus 0.1%. According to NMA, at week 4, difamilast 1% twice daily (BID) showed a significant improvement in the IGA score and percent EASI score change from baseline versus placebo; however, compared to other comparators, point estimates numerically favoured difamilast 1% but were not statistically significant. Difamilast 1% BID showed a significantly lower incidence of acne than delgocitinib 0.3% BID. There was no statistically significant difference in the incidence of serious AEs, acne, and skin infections compared to placebo or other comparators.
Conclusion: This study establishes the efficacy and safety of current topical treatment options and recently marketed delgocitinib and difamilast ointments for AD in Japan.
Keywords: Adverse event; Atopic dermatitis; Delgocitinib; Difamilast; EASI; IGA; Network meta-analysis; Systematic literature review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: This study and medical writing assistance were funded by Otsuka Pharmaceutical Co., Ltd. Hiroyuki Murota has received consulting fees and/or speaker honoraria from Maruho Co., Ltd., Kaken Pharmaceutical Co., Ltd., Sanofi K.K., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Kyowa Kirin Co., Ltd., Torii Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., AbbVie GK, and Otsuka Pharmaceutical Co., Ltd. Takeshi Nakahara has received consulting fees and/or speaker honoraria from Mitsubishi Tanabe Pharma Corp., Taiho Pharmaceutical Co., Ltd., Torii Pharmaceutical Co., Ltd., Maruho Co., Ltd., Sanofi K.K., AbbVie GK, Eli Lilly Japan K.K., Sun Pharma Japan Ltd., and Otsuka Pharmaceutical Co., Ltd. Miyuki Matsukawa, Hiroe Takeda, Tomohiro Kondo, and Kentaro Yamato are employees of Otsuka Pharmaceutical Co., Ltd. Xinyu Wang was employed by Otsuka Pharmaceutical Co., Ltd., during the conduct of the study and reports no conflicts of interest. Some of the authors are employees of Otsuka Pharmaceutical Co., Ltd., but the results were objectively evaluated using legitimate methods. Ethical Approval: This study is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Figures




References
-
- Torisu-Itakura H, Anderson P, Piercy J, Pike J, Sakamoto A, Kabashima K. Impact of itch and skin pain on quality of life in adult patients with atopic dermatitis in Japan: results from a real-world, point-in-time, survey of physicians and patients. Curr Med Res Opin. 2022;38(8):1401–10. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

