Targeting the Neonatal Fc Receptor in Autoimmune Diseases: Pipeline and Progress
- PMID: 40156757
- PMCID: PMC12031853
- DOI: 10.1007/s40259-025-00708-2
Targeting the Neonatal Fc Receptor in Autoimmune Diseases: Pipeline and Progress
Abstract
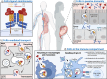
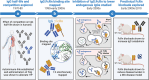
Autoimmune diseases are highly prevalent and affect people at all ages, women more often than men. The most prominent immunological manifestation is the production of antibodies directed against self-antigens. In many cases, these antibodies (Abs) drive the pathogenesis by attacking the body's own healthy cells, causing serious health problems that may be life threatening. Most autoantibodies are of the immunoglobulin G (IgG) isotype, which has a long plasma half-life and potent effector functions. Thus, there is a need for specific treatment options that rapidly eliminate these pathogenic IgG auto-Abs. In this review, we discuss how the neonatal Fc receptor (FcRn) acts as a regulator of the high levels of not only IgG Abs, but also albumin, by rescuing both these soluble proteins from cellular catabolism, and how a molecular and cellular understanding of this complex biology has spurred an intense interest in the development of FcRn-targeting strategies for the treatment of IgG-driven autoimmune diseases. We find that this emerging therapeutic class demonstrates efficacy within several autoimmune diseases with distinct pathophysiology. This offers hope for both new therapeutic avenues for highly prevalent diseases currently treated by other means, and rare diseases with no approved therapies to date. In addition, we elaborate on studies that have led to approval of the first FcRn antagonists, the clinical progress and structural design of molecules in the pipeline, their position in the overall therapeutic landscape of autoimmunity, the design of next-generation antagonists as well as the use of this receptor-targeting principle for other therapeutic applications.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by University of Oslo (incl Oslo University Hospital). This work was partially supported by the Research Council of Norway through its Centers of Excellence scheme, project number 332727, and its Proof-of-Concept Research commercialization program, project number 333902, as well as by the Novo Nordisk Foundation Pioneer Innovator Grant 0090894. Conflicts of Interest/Competing Interests: Torleif Tollefsrud Gjølberg, Simone Mester, Gaia Calamera, and Jenny Skjermo Telstad are employees of Authera AS. Torleif Tollefsrud Gjølberg, Simone Mester, Gaia Calamera, Jan Terje Andersen, and Inger Sandlie have ownership interests in Authera AS. Inger Sandlie and Jan Terje Andersen are inventors of the invention claimed in a patent family arising from WO2017158426 and WO2019057564 with the title “Engineered immunoglobulins with altered FcRn binding”. Ethics Approval: Not applicable. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Availability of Data and Material: Not applicable. Code Availability: Not applicable. Authors’ Contributions: TTG, SM, and JTA conceived the overall concept, contributed to the writing of the manuscript, conducted the literature search, and supervised the manuscript development. TTG performed the majority of the writing and data extraction. SM performed the majority of the work on graphical presentations and illustrations. GC and JST contributed to the writing of the manuscript. IS provided expert opinion and contributed to the writing of the manuscript.
Figures


Similar articles
-
FcRn inhibitors: Transformative advances and significant impacts on IgG-mediated autoimmune diseases.Autoimmun Rev. 2025 Feb 28;24(3):103719. doi: 10.1016/j.autrev.2024.103719. Epub 2024 Dec 11. Autoimmun Rev. 2025. PMID: 39672251 Review.
-
Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention.J Allergy Clin Immunol. 2020 Sep;146(3):467-478. doi: 10.1016/j.jaci.2020.07.015. J Allergy Clin Immunol. 2020. PMID: 32896307 Review.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
The neonatal Fc receptor as therapeutic target in IgG-mediated autoimmune diseases.Cell Mol Life Sci. 2010 Aug;67(15):2533-50. doi: 10.1007/s00018-010-0318-6. Epub 2010 Mar 9. Cell Mol Life Sci. 2010. PMID: 20217455 Free PMC article. Review.
-
The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy.Adv Drug Deliv Rev. 2015 Aug 30;91:109-24. doi: 10.1016/j.addr.2015.02.005. Epub 2015 Feb 19. Adv Drug Deliv Rev. 2015. PMID: 25703189 Free PMC article. Review.
References
-
- Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–95. - PubMed
-
- Wen X, Li B. A population-based study on autoimmune disease. Lancet. 2023;401(10391):1829–31. - PubMed
-
- Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

