Prothrombin Complex Concentrate vs Frozen Plasma for Coagulopathic Bleeding in Cardiac Surgery: The FARES-II Multicenter Randomized Clinical Trial
- PMID: 40156829
- PMCID: PMC11955085
- DOI: 10.1001/jama.2025.3501
Prothrombin Complex Concentrate vs Frozen Plasma for Coagulopathic Bleeding in Cardiac Surgery: The FARES-II Multicenter Randomized Clinical Trial
Abstract
Importance: Excessive bleeding is a common and prognostically important complication of cardiac surgery. For bleeding related to coagulation factor deficiency, frozen plasma is the most used therapy. Preliminary trials indicate that 4-factor prothrombin complex concentrate (PCC) may be a suitable alternative.
Objective: To compare the efficacy and safety of PCC with frozen plasma in patients undergoing cardiac surgery with coagulopathic bleeding.
Design, setting, and participants: Unblinded randomized noninferiority controlled clinical trial at 12 hospitals in Canada and the US involving adults (≥18 years) who had developed bleeding related to coagulation factor deficiency after termination of cardiopulmonary bypass during surgery (November 30, 2022, to May 28, 2024). Final 30-day follow-up visit was completed on June 28, 2024.
Intervention: A total of 265 patients were randomized to receive PCC (1500 IU ≤60 kg; 2000 IU >60 kg) and 263, frozen plasma (3 U ≤60 kg; 4 U >60 kg) in the operating room. A second dose was allowed over the next 24 hours if indicated; thereafter, only frozen plasma could be used.
Main outcomes and measures: The primary outcome was hemostatic response (effective if no hemostatic interventions occurred from 60 minutes to 24 hours after treatment initiation). The noninferiority of PCC vs frozen plasma was assessed using a 10% margin and a 1-sided α of .025, with subsequent testing for superiority if noninferiority was demonstrated. Secondary outcomes included allogeneic blood transfusions and adverse events. Patients were followed up until postoperative day 30.
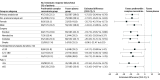
Results: Of 538 enrolled patients, 420 patients (median age, 66 years [IQR, 57-73 years]; 74%, male; 10%, Asian; 1%, Black; and 65%, White) were included in the primary analysis; of those, 296 (70%) underwent complex surgeries. Compared with the 207 patients in the frozen plasma group, the 213 patients in the PCC group had higher hemostatic effectiveness (166 [77.9%] vs 125 [60.4%]; difference, 17.6%; 95% CI, 8.7%-26.4%; P < .001 for noninferiority and superiority) and had received fewer transfusions including red blood cells, platelets, and noninvestigational frozen plasma units (mean, 6.6 units; 95% CI, 5.7-7.7 vs 9.3 units; 95% CI, 8.0-10.8; difference, 2.7; 95% CI, 1.0-4.4; P = .002). Seventy-seven patients (36.2%) in the PCC group vs 98 (47.3%) in the frozen plasma group experienced serious adverse events (relative risk [RR], 0.76; 95% CI, 0.61-0.96; P = .02). Twenty-two patients (10.3%) in the PCC group and 39 (18.8%) in the frozen plasma group had acute kidney injury (RR, 0.55; 95% CI, 0.34-0.89; P = .02).
Conclusions and relevance: In this unblinded randomized clinical trial, PCC had superior hemostatic efficacy and safety advantages to frozen plasma among patients requiring coagulation factor replacement for bleeding during cardiac surgery.
Trial registration: ClinicalTrials.gov Identifier: NCT05523297.
Conflict of interest statement
Figures

Comment in
-
Is It Time to Replace Plasma With Prothrombin Complex Concentrate in Cardiac Surgery?JAMA. 2025 May 27;333(20):1777-1778. doi: 10.1001/jama.2025.3644. JAMA. 2025. PMID: 40156830 No abstract available.
References
-
- Salenger R, Arora RC, Bracey A, et al. Cardiac surgical bleeding, transfusion, and quality metrics: joint consensus statement by the Enhanced Recovery After Surgery Cardiac Society and Society for the Advancement of Patient Blood Management. Ann Thorac Surg. 2025;119(2):280-295. doi: 10.1016/j.athoracsur.2024.06.039 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

