Unveiling spontaneous renal tubule-like structures from human adult renal progenitor cell spheroids derived from urine
- PMID: 40156847
- PMCID: PMC11954590
- DOI: 10.1093/stcltm/szaf002
Unveiling spontaneous renal tubule-like structures from human adult renal progenitor cell spheroids derived from urine
Abstract
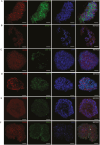
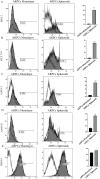
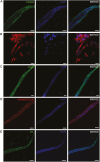
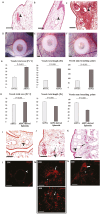
The rapidly developing field of renal spheroids and organoids has emerged as a valuable tool for modeling nephrotoxicity, kidney disorders, and kidney development. However, existing studies have relied on intricate and sophisticated differentiation protocols to generate organoids and tubuloids, necessitating the external administration of multiple growth factors within precise timeframes. In our study, we demonstrated that human adult renal progenitor cells (ARPCs) isolated from the urine of both healthy subjects and patients can form spheroids that naturally generated very long tubule-like structures. Importantly, the generation of these tubule-like structures is driven solely by ARPCs, without the need for the external use of chemokines or growth factors to artificially induce this process. These tubule-like structures exhibit the expression of structural and functional renal tubule markers and bear, in some cases, striking structural similarities to various nephron regions, including the distal convoluted tubule, the loop of Henle, and proximal convoluted tubules. Furthermore, ARPC spheroids express markers typical of pluripotent cells, such as stage-specific embryonic antigen 4 (SSEA4), secrete elevated levels of renin, and exhibit angiogenic properties. Notably, ARPCs isolated from the urine of patients with IgA nephropathy form spheroids capable of recapitulating the characteristic IgA1 deposition observed in this disease. These findings represent significant advancements in the field, opening up new avenues for regenerative medicine in the study of kidney development, mechanisms underlying renal disorders, and the development of regenerative therapies for kidney-related ailments.
Keywords: IgA nephropathy; regenerative medicine; renal progenitors; spheroids; tubule-like structures.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures



References
-
- Sallustio F, Serino G, Schena FP.. Potential reparative role of resident adult renal stem/progenitor cells in acute kidney injury. Biores Open Access. 2015;4:326-333. https://doi.org/ 10.1089/biores.2015.0011. - DOI - PMC - PubMed
-
- Ferenbach DA, Bonventre JV.. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264-276. https://doi.org/ 10.1038/nrneph.2015.3 - DOI - PMC - PubMed
-
- Maeshima A, Takahashi S, Nakasatomi M, Nojima Y.. Diverse cell populations involved in regeneration of renal tubular epithelium following acute kidney injury. Stem Cells Int. 2015;2015:1-8. https://doi.org/ 10.1155/2015/964849 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

