Preclinical development of a replication-competent vesicular stomatitis virus-based Lassa virus vaccine candidate advanced into human clinical trials
- PMID: 40157130
- PMCID: PMC11994357
- DOI: 10.1016/j.ebiom.2025.105647
Preclinical development of a replication-competent vesicular stomatitis virus-based Lassa virus vaccine candidate advanced into human clinical trials
Abstract
Background: Lassa fever (LF) is a zoonotic haemorrhagic disease caused by Lassa virus (LASV), which is endemic in West African countries. The multimammate rat is the main animal reservoir and its geographic range is expected to expand due to influences like climate change and land usage, and this will place larger parts of Africa at risk. We conducted preclinical development on a promising experimental vaccine that allowed its advancement into human trials.
Methods: The LF vaccine is based on a vesicular stomatitis virus (VSV) vector in which the VSV glycoprotein (G) was replaced with the LASV glycoprotein complex (GPC). Earlier studies showed that this vaccine (VSVΔG-LASV-GPC) was efficacious in macaques, thus we regenerated VSVΔG-LASV-GPC using laboratory and documentation practices required to support vaccine manufacturing and human trials. The efficacy of the clinical vaccine candidate was assessed in cynomolgus macaques and more extensive immunologic analysis was performed than previously to investigate immune responses associated with protection.
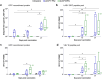
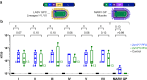
Findings: A single VSVΔG-LASV-GPC vaccination elicited innate, humoural and cellular immune responses, prevented development of substantial LASV viraemia, and protected animals from disease. Vaccinated macaques developed polyfunctional antibodies and serum was shown to neutralize virus expressing GPCs representative of geographically diverse LASV lineages.
Interpretation: The VSVΔG-LASV-GPC clinical candidate elicited immunity that protected 10 of 10 vaccinated macaques from disease supporting its use in a clinical development program, which recently progressed to phase 2 clinical trials. Moreover, immunologic analysis showed that virus-neutralizing serum antibodies likely played a role in preventing LASV disease in vaccinated macaques.
Funding: This work was supported by the Coalition for Epidemic Preparedness Innovations (CEPI), The National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), The Bill and Melinda Gates Global Vaccine Accelerator Program, the Burroughs Wellcome Fund, and financial gifts and support by Nancy Zimmerman, Mark and Lisa Schwartz, and Terry and Susan Ragon.
Keywords: Clinical vaccine candidate; Lassa virus; Neutralizing antibodies; Vesicular stomatitis virus vector.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests U.S. patent number 8,796,013 entitled “Pre- or Post-Exposure Treatment for Filovirus or Arenavirus Infection” issued to TWG on 5 August 2014 held by Boston University. GA is a founder/equity holder in Seromyx Systems and Leyden Labs. GA has served as a scientific advisor for Sanofi Vaccines. GA has collaborative agreements with GSK, Merck, Abbvie, Sanofi, Medicago, BioNtech, Moderna, BMS, Novavax, SK Biosciences, Gilead, and Sanaria. RPM has collaborative agreements with Abbvie, Sanofi, Moderna, and Pfizer. A U.S. provisional patent application (Serial No. 63/652,870) has been filed on the human vaccine candidate by IAVI (CLP, ES, MY, MBF).
Figures





References
-
- Raabe V., Mehta A.K., Evans J.D., Science Working Group of the National Emerging Special Pathogens T, Education Center Special Pathogens Research N Lassa virus infection: a summary for clinicians. Int J Infect Dis. 2022;119:187–200. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

