Characterization of a new mutation of mitochondrial ND6 gene in hepatocellular carcinoma and its effects on respiratory complex I
- PMID: 40157968
- PMCID: PMC11954883
- DOI: 10.1038/s41598-025-91746-x
Characterization of a new mutation of mitochondrial ND6 gene in hepatocellular carcinoma and its effects on respiratory complex I
Abstract
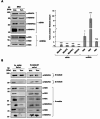
Hepatocellular carcinoma (HCC) is the most common form of liver cancer, which often arises from previous liver pathologies such as HBV, HCV, and alcohol abuse. It is typically associated with an enlarged cirrhotic organ. In this study, we analyzed tumor and distal tissues from a patient who underwent liver resection for HCC with no previous pathologies and whose liver showed normal function without signs of cirrhosis. Genetic analysis of mitochondrial DNA (mtDNA) revealed a novel variant of the gene encoding the NADH dehydrogenase subunit 6 (ND6) protein in the tumor tissue. The deletion of a thymidine generated an early stop codon, resulting in a truncated form of the protein (ΔND6) with 50% of the C-terminal primary sequence missing. ND6 is a subunit of the NADH dehydrogenase complex, also known as Complex I, the largest complex in the electron transport chain. Previous studies have linked mtDNA Complex I mutations to mitochondrial disorders and cancer. Through biochemical analyses, we characterized this new mutation and showed that the expression of ΔND6 negatively affects the stability and functionality of Complex I. Data were confirmed by molecular dynamics simulations suggesting conformational rearrangements, overall revealing a leading role of ND6 in the assembly of Complex I.
Keywords: Hepatocellular carcinoma; Mitochondria; Mitochondrial DNA; Molecular dynamics simulations.; ND6 gene mutation; Respiratory complex I assembly.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Reactive oxygen. Species (ROS) as pleiotropic physiological signalling agents |. Nat. Rev. Mol. Cell Biol.https://www.nature.com/articles/s41580-020-0230-3 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

