The specific contributions of factor H and factor I in controlling fluid phase activation of the alternative complement pathway
- PMID: 40158007
- PMCID: PMC11954907
- DOI: 10.1038/s41598-025-95533-6
The specific contributions of factor H and factor I in controlling fluid phase activation of the alternative complement pathway
Abstract
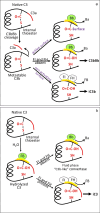
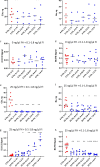
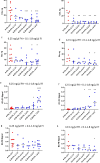
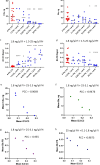
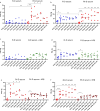
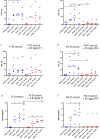
The immediate defense provided by the alternative complement pathway (AP) is under constant control by fluid phase regulators, factor H (FH) and factor I (FI), to prevent nonessential activation. Removal of either FI or FH from serum results in spontaneous AP activation, although the extent of activation caused by separate removal of each regulator has not been compared. In purified protein reactions with < 25% normal FH levels, additions of 6-100% normal FI levels, did not reduce C3a, Ba or FB cleavage. In reactions with 100% FH: C3a was not generated; Ba and FB cleavage was 3-fold lower; and C3 inactivation increased 2-fold as FI concentrations doubled. In reactions with 100% FI, FH levels ≥ 25% reduced C3a and Ba levels. AP activation levels were also compared in FI-depleted and FH-depleted serum. After magnesium addition to FI-depleted serum, C3a remained unchanged and Ba increased 3-fold, whereas in FH-depleted serum, C3a increased 13-fold and Ba increased 20-fold. Addition of 100% FI protein to FI-depleted serum did not change C3a and Ba, whereas, 100% FH added to FH-depleted serum prevented all activation. We conclude that normal levels of FH are sufficient to compensate for FI deficiencies and prevent unnecessary fluid phase AP activation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Pangburn, M. K. & Müller-Eberhard, H. J. Kinetic and thermodynamic analysis of the control of C3b by the complement regulatory proteins factors H and I. Biochemistry22, 178–185 (1983). - PubMed
-
- Pangburn, M. K., Pangburn, K. L., Koistinen, V., Meri, S. & Sharma, A. K. Molecular mechanisms of target recognition in an innate immune system: interactions among factor H, C3b, and target in the alternative pathway of human complement. J. Immunol.164, 4742–4751 (2000). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

