Oncogene-induced senescence mitochondrial metabolism and bioenergetics drive the secretory phenotype: further characterization and comparison with other senescence-inducing stimuli
- PMID: 40158257
- PMCID: PMC11997345
- DOI: 10.1016/j.redox.2025.103606
Oncogene-induced senescence mitochondrial metabolism and bioenergetics drive the secretory phenotype: further characterization and comparison with other senescence-inducing stimuli
Abstract
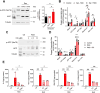
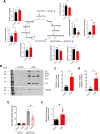
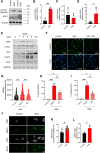
Cellular senescence is characterized by proliferation arrest and a senescence-associated secretory phenotype (SASP), that plays a role in aging and the progression of various age-related diseases. Although various metabolic alterations have been reported, no consensus exists regarding mitochondrial bioenergetics. Here we compared mitochondrial metabolism of human fibroblasts after inducing senescence with different stimuli: the oxidant hydrogen peroxide (H2O2), the genotoxic doxorubicin, serial passage, or expression of the H-RASG12V oncogene (RAS). In senescence induced by H2O2, doxorubicin or serial passage a decrease in respiratory control ratio (RCR) and coupling efficiency was noted, in relation to control cells. On the contrary, oncogene-induced senescent cells had an overall increase in respiration rates, RCR, spare respiratory capacity and coupling efficiency. In oncogene-induced senescence (OIS) the increase in respiration rates was accompanied by an increase in fatty acid catabolism, AMPK activation, and a persistent DNA damage response (DDR), that were not present in senescent cells induced by either H2O2 or doxorubicin. Inhibition of AMPK reduced mitochondrial oxygen consumption and secretion of proinflammatory cytokines in OIS. Assessment of enzymes involved in acetyl-CoA metabolism in OIS showed a 3- to 7.5-fold increase in pyruvate dehydrogenase complex (PDH), a 40% inhibition of mitochondrial aconitase, increased phosphorylation and activation of ATP-citrate lyase (ACLY), and inhibition of acetyl-CoA carboxylase (ACC). There was also a significant increase in expression and nuclear levels of the deacetylase sirtuin 6 (SIRT6). These changes can influence the sub-cellular distribution of acetyl-CoA and modulate protein acetylation reactions in the cytoplasm and nuclei. In fact, ACLY inhibition reduced histone 3 acetylation (H3K9Ac) in OIS and secretion of SASP components. In summary, our data show marked heterogeneity in mitochondrial energy metabolism of senescent cells, depending on the inducing stimulus, reveal new metabolic features of oncogene-induced senescent cells and identify AMPK and ACLY as potential targets for SASP modulation.
Keywords: Bioenergetics; Fatty acid oxidation; Mitochondria; RAS oncogene; Senescence; TCA cycle.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures






References
-
- Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., Gil J., Hara E., Krizhanovsky V., Jurk D., Maier A.B., Narita M., Niedernhofer L., Passos J.F., Robbins P.D., Schmitt C.A., Sedivy J., Vougas K., Von Zglinicki T., Zhou D., Serrano M., Demaria M. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. - DOI - PubMed
-
- Suryadevara V., Hudgins A.D., Rajesh A., Pappalardo A., Karpova A., Dey A.K., Hertzel A., Agudelo A., Rocha A., Soygur B., Schilling B., Carver C.M., Aguayo-Mazzucato C., Baker D.J., Bernlohr D.A., Jurk D., Mangarova D.B., Quardokus E.M., Enninga E.A.L., Schmidt E.L., Chen F., Duncan F.E., Cambuli F., Kaur G., Kuchel G.A., Lee G., Daldrup-Link H.E., Martini H., Phatnani H., Al-Naggar I.M., Rahman I., Nie J., Passos J.F., Silverstein J.C., Campisi J., Wang J., Iwasaki K., Barbosa K., Metis K., Nernekli K., Niedernhofer L.J., Ding L., Wang L., Adams L.C., Ruiyang L., Doolittle M.L., Teneche M.G., Schafer M.J., Xu M., Hajipour M., Boroumand M., Basisty N., Sloan N., Slavov N., Kuksenko O., Robson P., Gomez P.T., Vasilikos P., Adams P.D., Carapeto P., Zhu Q., Ramasamy R., Perez-Lorenzo R., Fan R., Dong R., Montgomery R.R., Shaikh S., Vickovic S., Yin S., Kang S., Suvakov S., Khosla S., Garovic V.D., Menon V., Xu Y., Song Y., Suh Y., Dou Z., Neretti N. SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 2024 doi: 10.1038/s41580-024-00738-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

