Correlation analysis of invasive disease-free survival and overall survival in a real-world population of patients with HR+/HER2- early breast cancer
- PMID: 40159245
- PMCID: PMC11955092
- DOI: 10.1002/cncr.35817
Correlation analysis of invasive disease-free survival and overall survival in a real-world population of patients with HR+/HER2- early breast cancer
Abstract
Background: Overall survival (OS) is the gold standard for assessing clinical benefit in oncology but requires extended follow-up to detect sufficient events. Invasive disease-free survival (iDFS) requires shorter follow-up times and is considered an objective and clinically meaningful end point in early breast cancer (EBC) trials. The authors assessed iDFS as a surrogate end point for OS in adjuvant HR+/HER2- EBC using real-world patient-level data.
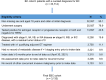
Methods: A retrospective analysis was conducted on patient data from the ConcertAI Patient360 database (January 1995-April 2021). Key inclusion criteria: age ≥18 years, stage II or III (AJCC 8th Edition) HR+/HER2- EBC, prior surgery, adjuvant endocrine therapy (ET). Spearman ρ, iterative multiple imputation ρ (IMI; 0.8-1 considered "very strong"), and R2 (clinical relevance R2 ≥ 0.70) were used to assess iDFS-OS relationship. Subgroup analyses included ET (nonsteroidal aromatase inhibitor or tamoxifen), stage, menopausal status, nodal status, prior (neo)adjuvant chemotherapy, and prior radiotherapy.
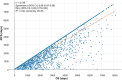
Results: A total of 3133 patients were included (1103 [35.2%] iDFS events; 554 [17.7%] OS events); mean age was 58.4 years, 98.8% were female, 29.9% were premenopausal, and 80.9% had stage II disease. Median follow-up time was 55.1 months. iDFS and OS exhibited a positive, very strong, clinically relevant correlation (Spearman ρ: 0.88 [0.87-0.89]; IMI ρ: 0.83 [0.79-0.86]; both p < .0001). iDFS accounted for 82% of variation in OS (R2 = 0.82). Results of all subgroup analyses were consistent with overall population.
Conclusions: This patient-level real-world analysis demonstrated very strong, positive correlations between iDFS and OS, supporting the use of iDFS as a reliable primary end point in adjuvant HR+/HER2- EBC.
Keywords: HR+/HER2– early breast cancer; correlation analysis; invasive disease‐free survival; overall survival; real world.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Stephanie L. Graff reports personal fees from Novartis, Pfizer, AstraZeneca, Genentech, Lilly, Daiichi Sankyo, Gilead Sciences, The Academy for Healthcare Learning, DAVA Oncology, MJH Life Sciences, WebMD/Medscape, IntegrityCE, MedPage Today, MedIQ, Medical Educator Consortium, and Research to Practice; stock ownership from HCA Healthcare; and research grants to institution from Daiichi Sankyo, Novartis, and AstraZeneca. Sara M. Tolaney reports grant funding to institution and personal fees from Eli Lilly, Novartis, AstraZeneca, Merck, Pfizer, Genentech/Roche, Bristol Myers Squibb, Eisai, Sanofi, Daiichi Sankyo, Gilead, Jazz Pharmaceuticals, and Menarini/Stemline; grant funding to institution from Exelixis, NanoString, and OncoPep; and personal fees from Seagen, Arvinas, Cullinan Oncology, eFFECTOR, CytomX, Sumitovant Biopharma, Natera, Tango Therapeutics, SystImmune, Hengrui USA, Blueprint Medicines, Reveal Genomics, Umoja Biopharma, Zentalis, Zymeworks, Circle Pharma, Bayer, Incyte Corp, and Aadi Bioscience. Lowell L. Hart reports grants to institution and personal fees from Novartis. Pedram Razavi reports institutional grants from Grail/Illumina, Novartis, AstraZeneca, Epic Sciences, Invitae/ArcherDX, Tempus, Inivata, and Biotheranostics; and personal fees from Novartis, AstraZeneca, Epic Sciences, Tempus, Inivata, Foundation Medicine, Pfizer, Daiichi Sankyo, Natera, Odyssey Biosciences, Paige.AI, and SAGA Diagnostics. Wolfgang Janni reports personal fees from Amgen, AstraZeneca, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Roche, Seagen, and Gilead; employment from Universitätsklinikum Ulm; speaker fees to institution from Novartis, GSK, Sanofi, Amgen, Roche, and Lilly; and serves as chair of AGO Breast Council. Lee S. Schwartzberg reports personal fees from AstraZeneca, Daiichi Sankyo, Genentech, Novartis, Pfizer, Seagen, Merck, Spectrum, Napo, and Amgen. Andriy Danyliv reports employment and stock ownership from Novartis. Murat Akdere reports employment and stock ownership from Novartis. Ilia Ferrusi reports employment and stock ownership from Novartis. Rishi Rajat Adhikary reports employment from Novartis. Joyce A. O’Shaughnessy reports personal fees from AbbVie, Agendia, Amgen, Aptitude, AstraZeneca, Bristol Myers Squibb, Celgene, Eisai, G1 Therapeutics, Genentech, Immunome, Ipsen Biopharmaceuticals, Lilly, Merck, Myriad, Novartis, Odonate Therapeutics, Pfizer, Puma, Prime, Roche, Seagen, Syndax, Carrick Therapeutics, Daiichi Sankyo, Gilead Sciences, Ontada, Pierre Fabre, Samsung, and Sanofi.
Figures
References
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V2.2025.
-
- de Gramont A, Hubbard J, Shi Q, et al. Association between disease‐free survival and overall survival when survival is prolonged after recurrence in patients receiving cytotoxic adjuvant therapy for colon cancer: simulations based on the 20,800 patient ACCENT data set. J Clin Oncol. 2010;28(3):460‐465. doi:10.1200/jco.2009.23.1407 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

