Mode of Death in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction: The FINEARTS-HF Randomized Clinical Trial
- PMID: 40159247
- PMCID: PMC11955905
- DOI: 10.1001/jamacardio.2025.0860
Mode of Death in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction: The FINEARTS-HF Randomized Clinical Trial
Abstract
Importance: The mode of death in patients with heart failure with mildly reduced ejection fraction (HFmrEF) or heart failure with preserved ejection fraction (HFpEF) remains poorly understood and may vary by EF.
Objective: To evaluate the mode of death according to EF and the treatment effect of finerenone on cause-specific mortality in patients with HFmrEF/HFpEF.
Design, setting, and participants: This was a prespecified secondary analysis of the Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients With Heart Failure (FINEARTS-HF) randomized clinical trial, which evaluated clinical outcomes in 6001 patients with HF and EF greater than or equal to 40% randomly assigned to finerenone or placebo. The mode of death in relation to baseline EF categories (<50%, ≥50-<60%, and ≥60%) was examined, and the effect of randomized treatment on cause-specific death in Cox regression models was assessed. Data analysis was conducted between September 2024 and January 2025.
Interventions: Finerenone vs placebo.
Main outcomes and measures: Mode of death as centrally adjudicated by a clinical end points committee.
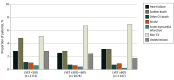
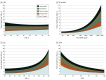
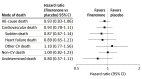
Results: Of 1013 patients (16.9%; median [IQR] age, 76 [69-82] years; 594 male [58.6%]) who died during median (IQR) follow-up of 32 (23-36) months, mode of death was ascribed to cardiovascular causes in 502 (49.6%), noncardiovascular causes in 368 (36.3%), and undetermined cause in 143 (14.1%). Of cardiovascular deaths, 215 (42.8%) were due to sudden death, 163 (32.4%) to HF, 48 (9.6%) to stroke, 25 (5.0%) to myocardial infarction, and 51 (10.2%) to other cardiovascular causes. The proportion of all-cause, cardiovascular, and sudden death was higher in those with EF less than 50%. The proportion of deaths related to HF was similar across EF categories, and the proportion of deaths due to myocardial infarction, stroke, and other cardiovascular causes was low regardless of EF. Randomization to finerenone did not significantly reduce death or cause-specific death compared with placebo in any EF category.
Conclusions and relevance: Among patients with HFmrEF/HFpEF in the FINEARTS-HF randomized clinical trial, higher proportions of cardiovascular and overall mortality in those with EF less than 50% were related principally to higher proportions of sudden death. A clear treatment effect of finerenone on cardiovascular or cause-specific mortality was not identified, although the trial was likely underpowered for these outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT04435626.
Conflict of interest statement
Figures
Similar articles
-
Finerenone for Heart Failure and Risk Estimated by the PREDICT-HFpEF Model: A Secondary Analysis of FINEARTS-HF.JAMA Cardiol. 2025 Jun 1;10(6):535-544. doi: 10.1001/jamacardio.2025.0025. JAMA Cardiol. 2025. PMID: 40042880 Clinical Trial.
-
Finerenone in Heart Failure With Improved Ejection Fraction: The FINEARTS-HF Randomized Clinical Trial.JAMA Cardiol. 2025 Jul 1;10(7):740-745. doi: 10.1001/jamacardio.2025.1101. JAMA Cardiol. 2025. PMID: 40397470 Free PMC article. Clinical Trial.
-
Finerenone and Atrial Fibrillation in Heart Failure: A Secondary Analysis of the FINEARTS-HF Randomized Clinical Trial.JAMA Cardiol. 2025 Jul 1;10(7):696-707. doi: 10.1001/jamacardio.2025.0848. JAMA Cardiol. 2025. PMID: 40156827 Free PMC article. Clinical Trial.
-
[Mineralocorticoid receptor antagonists in heart failure with preserved/mildly reduced ejection fraction: from TOPCAT to FINEARTS-HF].G Ital Cardiol (Rome). 2025 Jan;26(1):38-49. doi: 10.1714/4394.43958. G Ital Cardiol (Rome). 2025. PMID: 39714499 Review. Italian.
-
Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction.Cochrane Database Syst Rev. 2018 Jun 28;6(6):CD012721. doi: 10.1002/14651858.CD012721.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2021 May 22;5:CD012721. doi: 10.1002/14651858.CD012721.pub3. PMID: 29952095 Free PMC article. Updated.
References
-
- Solomon SD, Wang D, Finn P, et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110(15):2180-2183. doi: 10.1161/01.CIR.0000144474.65922.AA - DOI - PubMed
-
- Desai AS, Jhund PS, Claggett BL, et al. Effect of dapagliflozin on cause-specific mortality in patients with heart failure across the spectrum of ejection fraction: a participant-level pooled analysis of DAPA-HF and DELIVER. JAMA Cardiol. 2022;7(12):1227-1234. doi: 10.1001/jamacardio.2022.3736 - DOI - PMC - PubMed
-
- Kosiborod MN, Deanfield J, Pratley R, et al. ; SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM Trial Committees and Investigators . Semaglutide vs placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomized trials. Lancet. 2024;404(10456):949-961. doi: 10.1016/S0140-6736(24)01643-X - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

