Upfront intensive treatment analysis of the Italian Cohort Study on FLT3-mutated AML patients (FLAM): The impact of a FLT3 inhibitor addition to standard chemotherapy in the real-life setting
- PMID: 40159434
- PMCID: PMC11955083
- DOI: 10.1002/cncr.35824
Upfront intensive treatment analysis of the Italian Cohort Study on FLT3-mutated AML patients (FLAM): The impact of a FLT3 inhibitor addition to standard chemotherapy in the real-life setting
Abstract
Background: The addition of a FLT3 inhibitor (FLT3i) to standard chemotherapy to treat fit newly diagnosed (ND) patients with FLT3-mutated acute myeloid leukemia (AML) represents the standard of care resulting from clinical trial results. However, evidence regarding FLT3i adoption in routine clinical practice is still scarce.
Methods: Clinical data are reported from 394 ND patients with FLT3-mutated AML enrolled in the retrospective observational Italian Cohort Study on FLT3-mutated patients with AML and treated with an upfront intensive regimen with (FLT3i group, n = 92) or without (CT group, n = 302) the addition of a FLT3i.
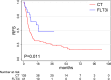
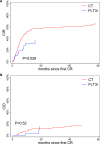
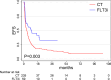
Results: With a median follow-up time of 34.5 months, an effectiveness benefit obtained by FLT3i incorporation both in terms of overall survival (median, 34.9 in the FLT3i vs 12.7 months in the CT group, p < .01) and relapse-free survival (median, 18.9 in the FLT3i vs 7.6 months in the CT group, p = .01) was documented, with a higher composite complete remission rate (75.4% in the FLT3i vs 62.4% in the CT group, p = .052). FLT3i benefit seemed to be independent from the transplant rate.
Conclusions: In conclusion, the benefit of FLT3i addition to upfront intensive treatment in newly diagnosed FLT3-mutated AML patients was confirmed in a large, real-life cohort study.
Keywords: FLT3 gene mutation; FLT3 inhibitor; acute myeloid leukemia; intensive treatment; real‐life setting; standard chemotherapy.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
GM: Consultant/speaker bureau of Abbvie, Astellas, AstraZeneca, Immunogen, Jansenn, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, and Takeda; research support from Abbvie, Astellas, AstraZeneca, Daiichi Sankyo, Pfizer, and Syros. CP: Honoraria of Astellas and Novartis, Advisory Board of Astellas and Novartis. All other authors have no conflicts of interest to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

