Transcriptional Co-Activator With PDZ Binding Motif (TAZ) Inhibits Dexamethasone-Induced Muscle Atrophy via mTOR Signalling
- PMID: 40159627
- PMCID: PMC11955411
- DOI: 10.1002/jcsm.13790
Transcriptional Co-Activator With PDZ Binding Motif (TAZ) Inhibits Dexamethasone-Induced Muscle Atrophy via mTOR Signalling
Abstract
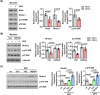
Background: Glucocorticoid therapy has a beneficial effect in several diseases, but chronic treatment has adverse effects, including muscle atrophy, which refers to the gradual decrease in muscle mass, size and strength. It is important to know how the muscle atrophy occurs, but the underlying mechanism is not yet fully understood. This study shows that dexamethasone decreases levels of the transcriptional co-activator with PDZ binding motif (TAZ), which facilitates dexamethasone-induced muscle atrophy.
Methods: To induce muscle atrophy, C2C12 myotubes were treated with dexamethasone, and mice were fed with water containing dexamethasone. Muscle atrophy was analysed for the expression of myosin heavy chain, MuRF1 and Atrogin-1 using immunofluorescence staining, immunoblot analysis and qRT-PCR. Muscle tissue was analysed by haematoxylin and eosin staining. Adeno-associated virus was used for overexpression of wild-type and mutant TAZ.
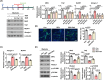
Results: TAZ levels decrease in dexamethasone-treated mice (0.36-fold, p < 0.001) and C2C12 myotubes (0.44-fold, p = 0.024). Overexpression of the TAZ mutant, which resists its proteolytic degradation, inhibits dexamethasone-induced muscle atrophy. Atrogin-1 and MuRF1 interact with TAZ and facilitate its degradation in dexamethasone-treated C2C12 myotubes. TAZ mutant stimulates protein synthesis through activation of mTOR signalling via induction of RhebL1 (DEX; Con vs, TAZ4SA: 5.1-fold, p < 0.001) in dexamethasone-treated mice. Ginsenoside Rb3 increases TAZ levels in dexamethasone-treated mice (1.49-fold, p = 0.007) and C2C12 myotubes (1.63-fold, p = 0.01), which stimulates mTOR signalling and inhibits dexamethasone-induced muscle atrophy.
Conclusions: Our results demonstrate a novel regulatory mechanism of dexamethasone-induced muscle atrophy by TAZ, suggesting that stabilisation of TAZ in muscle cells ameliorates the muscle atrophy. These results suggest that TAZ may be a drug target for the dexamethasone-induced muscle atrophy.
Keywords: dexamethasone; ginsenoside; mTOR; muscle atrophy.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest..
Figures




References
-
- Cohen S., Nathan J. A., and Goldberg A. L., “Muscle Wasting in Disease: Molecular Mechanisms and Promising Therapies,” Nature Reviews. Drug Discovery 14 (2015): 58–74. - PubMed
-
- Shimizu N., Yoshikawa N., Ito N., et al., “Crosstalk Between Glucocorticoid Receptor and Nutritional Sensor mTOR in Skeletal Muscle,” Cell Metabolism 13 (2011): 170–182. - PubMed
-
- Bodine S. C., Latres E., Baumhueter S., et al., “Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy,” Science 294 (2001): 1704–1708. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

