Migration Dynamics of Human NK Cell Preparations in Microchannels and Their Invasion Into Patient-Derived Tissue
- PMID: 40159644
- PMCID: PMC11955413
- DOI: 10.1111/jcmm.70481
Migration Dynamics of Human NK Cell Preparations in Microchannels and Their Invasion Into Patient-Derived Tissue
Abstract
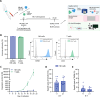
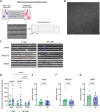
Natural killer (NK) cells are characterised by their ability to attack cancer cells without prior antigen stimulation. Additionally, clinical trials revealed great potential of NK cells expressing chimeric antigen receptors (CARs). Successful anti-tumour efficacy remains limited by migration and infiltration to the tumour site by NK cell preparations, which is linked to the scarcity in the knowledge of migration dynamics and invasion potential. Here, we applied a recently reported innovative microfluidic microchannel technology to gain insight into the intrinsic motility of NK cells. We assessed the baseline activated and proliferating NK cells in direct comparison with T cells and investigated their motility patterns in the presence of tumour cells. Additionally, we performed high-resolution 4D confocal imaging in patient-derived hyperplastic lymphatic tissues to assess their invasive capacity. Our data revealed that the invasion potential of NK cells was greater than that of T cells, despite their similar velocities. The flexibility of the NK cell nucleus may have contributed to the higher invasion potential. The motility of CD19-CAR-NK cell preparations was similar to that of non-transduced NK cells in hyperplastic lymphoid tissue, with improved targeted migration in tumour tissue, suggesting the suitability of genetically engineered NK cells for difficult-to-reach tumour tissues.
Keywords: CAR‐NK; DLBCL tissue; NK cells; T cells; hyperplastic lymphoid tissue; microchannel; motility.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
E.U. has a sponsored research project with Gilead and BMS and acts as a medical advisor for Phialogics and CRIION. The remaining authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

