Unveiling the Angiogenic Potential and Functional Decline of Valve Interstitial Cells During Calcific Aortic Valve Stenosis Progression
- PMID: 40159645
- PMCID: PMC11955408
- DOI: 10.1111/jcmm.70511
Unveiling the Angiogenic Potential and Functional Decline of Valve Interstitial Cells During Calcific Aortic Valve Stenosis Progression
Abstract
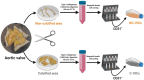
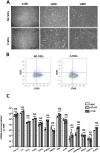
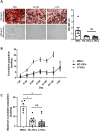
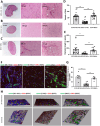
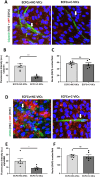
Valve interstitial cells (VICs) play a critical role in aortic valve calcification and angiogenic processes associated with calcific aortic valve stenosis (CAVS). Within the same valve, VICs from differently calcified regions can exhibit diverse phenotypic and functional properties. We hypothesised that VICs isolated from noncalcified (NC-VICs) and calcified (C-VICs) areas of human aortic valves possess distinct angiogenic characteristics. In this study, we isolated C-VICs and NC-VICs from 23 valves obtained after aortic valve replacement due to CAVS. Both VIC types exhibited similar phenotypes in culture, characterised by morphology, expression of mesenchymal/fibroblastic markers, proliferation and osteogenic differentiation. No significant differences were observed in the secretion of angiogenic factors, including VEGF-A, Ang-1, Ang-2, PlGF, bFGF between NC-VICs and C-VICs. However, when co-injected with endothelial colony-forming cells (ECFCs) into Matrigel implants in vivo in mice, implants containing NC-VICs showed significantly higher microvessel density compared to those with C-VICs (p < 0.001). Additionally, NC-VICs co-cultured with ECFCs expressed significantly higher levels of the perivascular markers αSMA and calponin compared to C-VICs (p < 0.001 and p < 0.05, respectively). In conclusion, our study reveals the heterogeneity in VIC plasticity within the aortic valve during CAVS. The diminished capacity of VICs from calcified areas to differentiate into perivascular cells suggests a loss of function as valve disease progresses. Furthermore, the ability of VICs to undergo perivascular differentiation may provide insights into valve homeostasis, angiogenesis and the exacerbation of calcification.
Keywords: aortic valve stenosis; endothelial progenitor cells; neovascularisation; perivascular cells; valve interstitial cell.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Yadgir S., Johnson C. O., Aboyans V., et al., “Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017,” Circulation 141 (2020): 1670–1680. - PubMed
-
- Syväranta S., Helske S., Laine M., et al., “Vascular Endothelial Growth Factor–Secreting Mast Cells and Myofibroblasts,” Arteriosclerosis, Thrombosis, and Vascular Biology 30 (2010): 1220–1227. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

