Status and outlook of solid electrolyte membrane reactors for energy, chemical, and environmental applications
- PMID: 40160366
- PMCID: PMC11951168
- DOI: 10.1039/d4sc08300h
Status and outlook of solid electrolyte membrane reactors for energy, chemical, and environmental applications
Abstract
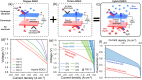
Solid electrolyte membrane reactors (SEMRs) can be operated at high temperatures with distinct reaction kinetics, or at lower temperatures (300-500 °C) for industrially relevant energy applications (such as solid oxide fuel/electrolysis cells, direct carbon fuel cells, and metal-air batteries), chemical (such as alkane dehydrogenation, C-C coupling, and NH3 synthesis), environmental (De-NO x , CO2 utilization, and separation), as well as their combined (one-step coupled CO2/H2O co-electrolysis and methanation reaction, power and chemical cogeneration) applications. SEMRs can efficiently integrate electrical, chemical, and thermal energy sectors, thereby circumventing thermodynamic constraints and production separation issues. They offer a promising way to achieve carbon neutrality and improve chemical manufacturing processes. This review thoroughly examines SEMRs utilizing various ionic conductors, namely O2-, H+, and hybrid types, with operations in different reactor/cell architectures (such as panel, tubular, single chamber, and porous electrolytes). The reactors operate in various modes including pumping, extraction, reversible, or electrical promoting modes, providing multiple functionalities. The discussion extends to examining critical materials for solid-state cells and catalysts essential for specific technologically important reactions, focusing on electrochemical performance, conversion efficiency, and selectivity. The review also serves as a first attempt to address the potential of process-intensified SEMRs through the integration of photo/solar, thermoelectric, and plasma energy and explores the unique phenomenon of electrochemical promotion of catalysis (EPOC) in membrane reactors. The ultimate goal is to offer insight into ongoing critical scientific and technical challenges like durability and operational cost hindering the widespread industrial implementation of SEMRs while exploring the opportunities in this rapidly growing research domain. Although still in an early stage with limited demonstrations and applications, advances in materials, catalysis science, solid-state ionics, and reactor design, as well as process intensification and/or system integration will fill the gaps in current high temperature operation of SEMRs and industrially relevant applications like sustainable clean chemical production, efficient energy conversion/storage, as well as environmental enhancement.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




























References
-
- Zheng T. Ou M. Xu S. Mao X. Wang S. He Q. Recent Progress of Bifunctional Electrocatalysts for Oxygen Electrodes in Unitized Regenerative Fuel Cells. J. Electrochem. 2023;29:2205301.
-
- McIntosh S. Gorte R. J. Direct hydrocarbon solid oxide fuel cells. Chem. Rev. 2004;104:4845–4865. - PubMed
-
- Schiffer Z. J. Manthiram K. Electrification and Decarbonization of the Chemical Industry. Joule. 2017;1:10–14.
Publication types
LinkOut - more resources
Full Text Sources

