Synthesis, Biotransformation, Characterization, and DFT Study of Organic Azachalcone Dyes and Secondary Metabolites with Biological and Conformation Dependence of Dipolar-Octupolar NLO Responses
- PMID: 40160730
- PMCID: PMC11947782
- DOI: 10.1021/acsomega.4c09074
Synthesis, Biotransformation, Characterization, and DFT Study of Organic Azachalcone Dyes and Secondary Metabolites with Biological and Conformation Dependence of Dipolar-Octupolar NLO Responses
Abstract
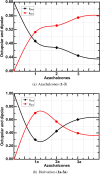
Chalcones are organic chromophores with diverse biological applications and potential for use in various electronic devices due to their recognized optical properties. This research focuses on the organic synthesis, FT-NMR characterization, and biotransformation of three azachalcones (1-3) using the Exserohilum rostratum fungus, yielding novel compounds (1a-3a). In vitro biological assays against Gram-positive and Gram-negative bacteria revealed promising pharmacological potential for these new chromophores. A key structural difference, the interchange of an HC = CH bond by a H2C-CH2 bond, significantly impacts biological and electronic properties. For instance, while biotransformed 1a exhibits similar activity to tetracycline and amoxicillin, compounds 2a and 3a demonstrate a 4-fold and thirty-fold increase in inhibitory activity against Gram-negative E. coli, respectively, compared to their parent compounds. Density functional theory calculations suggest that the biotransformation reaction reduces the refractive index (n), which may limit its applicability in certain light-handling applications. However, Hyper-Rayleigh scattering calculations indicate that these chromophores exhibit higher nonlinear optical (NLO) responses compared to standard NLO materials such as urea and p-nitroaniline, making them promising candidates for photonic and optoelectronic devices, such as nanostructured circuits. Interestingly, while the original molecules exhibit a dominant dipolar (Φ J=1) NLO response, the biotransformed compounds, as stable isomers, display a predominant octupolar (Φ J=3) architecture. These findings highlight the potential of these novel compounds for biotechnological and optoelectronic applications.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wang F.; Jiang J.; Hu S.; Hao X.; Cai Y. S.; Ye Y.; Ma H.; Sun W.; Cheng L.; Huang C.; Zhu H.; Zhang H.; Zhang G.; Zhang Y. Nidulaxanthone A, a xanthone dimer with a heptacyclic 6/6/6/6/6/6/6 ring system from Aspergillus sp.-F029. Org. Chem. Front. 2020, 7, 953–959. 10.1039/d0qo00113a. - DOI
-
- Schneider T.Nonlinear Optics in Telecommunications; Springer: Berlin, Heidelberg, 2004.
-
- Moloney J.Nonlinear Optics; CRC Press, Taylor & Francis Group: Boca Raton, FL, 2018.
-
- Loughlin W. A. Biotransformations in organic synthesis. Bioresour. Technol. 2000, 74, 49–62. 10.1016/S0960-8524(99)00145-5. - DOI
LinkOut - more resources
Full Text Sources
