Transcriptional signatures associated with the survival of Escherichia coli biofilm during treatment with plasma-activated water
- PMID: 40161322
- PMCID: PMC11952861
- DOI: 10.1016/j.bioflm.2025.100266
Transcriptional signatures associated with the survival of Escherichia coli biofilm during treatment with plasma-activated water
Abstract
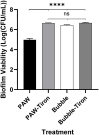
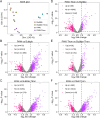
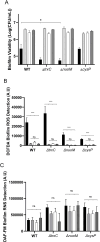
Biofilm formation on surfaces, tools and equipment can damage their quality and lead to high repair or replacement costs. Plasma-activated water (PAW), a new technology, has shown promise in killing biofilm and non-biofilm bacteria due to reactive oxygen and nitrogen species (RONS), particularly superoxide. However, the exact genetic mechanisms behind PAW's effectiveness against biofilms remain unclear. Here, we examined the stress responses of Escherichia coli biofilms exposed to sub-lethal PAW treatment using bulk RNA sequencing and transcriptomics. We compared gene expression in PAW-treated E. coli biofilms with and without superoxide removal, achieved by adding the scavenger Tiron. Biofilms treated with PAW exhibited a 40 % variation in gene expression compared to those treated with PAW-Tiron and controls. Specifically, PAW treatment resulted in 478 upregulated genes (>1.5 log2FC) and 186 downregulated genes (<-1.5 log2FC) compared to the control. Pathway and biological process enrichment analysis revealed significant upregulation of genes involved in sulfur metabolism, ATP-binding transporter, amino acid metabolism, hypochlorite response systems and oxidative phosphorylation in PAW-treated biofilms compared to control. Biofilm viability and intracellular RONS accumulation were tested for E. coli mutants lacking key genes from these pathways. Knockout mutants of thioredoxin (trxC), thiosulfate-binding proteins (cysP), and NADH dehydrogenase subunit (nuoM) showed significantly reduced biofilm viability after PAW treatment. Notably, ΔtrxC biofilms had the highest intracellular ROS accumulation, as revealed by 2',7'-dichlorofluorescin diacetate staining after PAW treatment. This confirms the importance of these genes in managing oxidative stress caused by PAW and highlights the significance of superoxide in PAW's bactericidal effects. Overall, our findings shed light on the specific genes and pathways that help E. coli biofilms survive and respond to PAW treatment, offering a new understanding of plasma technology and its anti-biofilm mechanisms.
Keywords: Biofilm; Escherichia coli; Plasma-activated water; Steel surfaces; Transcriptomics.
© 2025 Published by Elsevier B.V.
Conflict of interest statement
PJ Cullen is the CTO of PlasmaLeap Technologies, the supplier of the plasma power source and BSD reactor utilised in this study.
Figures
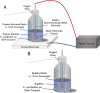

References
-
- Lopes F., Morin P., Oliveira R., Melo L. Interaction of Desulfovibrio desulfuricans biofilms with stainless steel surface and its impact on bacterial metabolism. J Appl Microbiol. 2006;101(5):1087–1095. - PubMed
-
- Dula S., Ajayeoba T.A., Ijabadeniyi O.A. Bacterial biofilm formation on stainless steel in the food processing environment and its health implications. Folia Microbiol. 2021;66(3):293–302. - PubMed
-
- Xia B., Vyas H.K.N., Zhou R., Zhang T., Hong J., Rothwell J.G., Rice S.A., Carter D., Ostrikov K., Cullen P.J., Mai-Prochnow A. The importance of superoxide anion for Escherichia coli biofilm removal using plasma-activated water. J Environ Chem Eng. 2023;11(3)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

