EGCG's anticancer potential unveiled: triggering apoptosis in lung cancer cell lines through in vitro investigation
- PMID: 40161336
- PMCID: PMC11954466
- DOI: 10.7717/peerj.19135
EGCG's anticancer potential unveiled: triggering apoptosis in lung cancer cell lines through in vitro investigation
Abstract
Background: Novel treatment techniques are needed since lung cancer is still a major worldwide health concern. Green tea contains a component called epigallocatechin-3-gallate (EGCG), which has demonstrated potential anticancer properties. This work sought to understand how EGCG affects the phosphatidylinositol-3-kinase protein kinase B (PI3K/Akt) signaling pathway, which in turn causes apoptosis in H1299 lung cancer cells.
Methods: In this experiment, multiple dosages of EGCG were applied to five H1299 cells and five A549 cell lines for a duration of 72 h. Apoptotic pathways, cellular responses, and protein expression levels were investigated in relation to EGCG by morphological, biochemical, and proliferation/migration investigations.
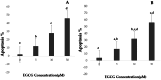
Results: In H1299 and A549 cells, EGCG raised apoptosis rates and, in a dose-dependent way, hindered cell growth. The levels of phosphorylated Akt (p-Akt) and PI3K (p-PI3K) dramatically reduced following EGCG administration, despite no significant alterations in Akt and PI3K expressions. These results imply that EGCG inhibits the activation of the PI3K/Akt signaling pathway, which in turn causes apoptosis in H1299 and A549 cells.
Conclusion: The research provides insights into the effects of EGCG on proliferation and migratory inhibition, as well as highlighting its potential to induce apoptosis in lung cancer cells. These results support EGCG's promise as a therapeutic agent in the treatment of lung cancer and further our understanding of the processes underlying its anticancer activities.
Keywords: A549 cells; Apoptosis rate; Cell proliferation; Epigallocatechin -3- gallate (EGCG); H1299 cell; Lung cancer; Protein expression.
©2025 Khair et al.
Conflict of interest statement
Luca Testarelli is an Academic Editor for PeerJ.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

