Tonic-clonic seizures induce hypersomnia and suppress rapid eye movement sleep in mouse models of epilepsy
- PMID: 40161404
- PMCID: PMC11954448
- DOI: 10.1093/sleepadvances/zpaf009
Tonic-clonic seizures induce hypersomnia and suppress rapid eye movement sleep in mouse models of epilepsy
Abstract
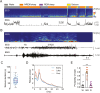
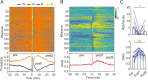
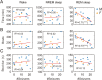
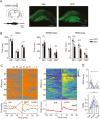
The reciprocal relationship between sleep and epilepsy has been reported by numerous clinical studies. However, the underlying neural mechanisms are poorly understood. Animal models of epilepsy are powerful tools to tackle this question. A lagging research area is the understudied sleep in epilepsy models. Here, we characterize sleep architecture and its relationship with seizures in a mouse model of sleep-related hypermotor epilepsy, caused by mutation of KCNT1. We demonstrated that nocturnal tonic-clonic seizures induce more non-rapid eye movement (NREM) sleep but suppress rapid eye movement (REM) sleep, resulting in altered sleep architecture in this mouse model. Importantly, the seizure number is quantitatively anticorrelated with the amount of REM sleep. Strikingly, this modulation of NREM and REM sleep states can be repeated in another mouse model of epilepsy with diurnal tonic-clonic seizures. Together, our findings provide evidence from rodent models to substantiate the close interplay between sleep and epilepsy, which lays the ground for mechanistic studies.
Keywords: EEG; Kcnt1; Sleep; epilepsy; tonic-clonic seizures.
© The Author(s) 2025. Published by Oxford University Press on behalf of Sleep Research Society.
Figures


Similar articles
-
Sleep-related hypermotor epilepsy activated by rapid eye movement sleep.Epileptic Disord. 2018 Feb 1;20(1):65-69. doi: 10.1684/epd.2017.0943. Epileptic Disord. 2018. PMID: 29171396
-
Influence of sleep on seizures and interictal epileptiform discharges in epilepsy.Encephalitis. 2025 Jan;5(1):1-5. doi: 10.47936/encephalitis.2024.00087. Epub 2024 Nov 12. Encephalitis. 2025. PMID: 39527944 Free PMC article.
-
Case report: A young man with non-rapid eye movement parasomnias in a KCNT1-related epilepsy family.Front Neurol. 2023 Nov 24;14:1280348. doi: 10.3389/fneur.2023.1280348. eCollection 2023. Front Neurol. 2023. PMID: 38073640 Free PMC article.
-
Sleep and arousal mechanisms in experimental epilepsy: epileptic components of NREM and antiepileptic components of REM sleep.Ment Retard Dev Disabil Res Rev. 2004;10(2):117-21. doi: 10.1002/mrdd.20022. Ment Retard Dev Disabil Res Rev. 2004. PMID: 15362167 Review.
-
Sleep Related Epilepsy and Pharmacotherapy: An Insight.Front Pharmacol. 2018 Sep 27;9:1088. doi: 10.3389/fphar.2018.01088. eCollection 2018. Front Pharmacol. 2018. PMID: 30319421 Free PMC article. Review.
References
-
- Krutoshinskaya Y, Coulehan K, Pushchinska G, Spiegel R.. The reciprocal relationship between sleep and epilepsy. J Pers Med. 2024;14(1):118. doi: https://doi.org/10.3390/jpm14010118 - DOI - PMC - PubMed
-
- Derry CP, Duncan S.. Sleep and epilepsy. Epilepsy Behav. 2013;26(3):394–404. doi: https://doi.org/10.1016/j.yebeh.2012.10.033 - DOI - PubMed
-
- Nobili L, Frauscher B, Eriksson S, et al.Sleep and epilepsy: a snapshot of knowledge and future research lines. J Sleep Res. 2022;31(4):e13622. doi: https://doi.org/10.1111/jsr.13622 - DOI - PMC - PubMed
-
- Roliz AH, Kothare S.. The interaction between sleep and epilepsy. Curr Neurol Neurosci Rep. 2022;22(9):551–563. doi: https://doi.org/10.1007/s11910-022-01219-1 - DOI - PubMed
-
- Lanigar S, Bandyopadhyay S.. Sleep and epilepsy: a complex interplay. Mo Med. 2017;114(6):453–457. doi: https://doi.org/10.1212/wnl.23.12.1335 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

