This is a preprint.
Focal Infrared Neural Stimulation Propagates Dynamical Transformations in Auditory Cortex
- PMID: 40161605
- PMCID: PMC11952546
- DOI: 10.1101/2025.03.12.642906
Focal Infrared Neural Stimulation Propagates Dynamical Transformations in Auditory Cortex
Abstract
Significance: Infrared neural stimulation (INS) has emerged as a potent neuromodulation technology, offering safe and focal stimulation with superior spatial recruitment profiles compared to conventional electrical methods. However, the neural dynamics induced by INS stimulation remain poorly understood. Elucidating these dynamics will help develop new INS stimulation paradigms and advance its clinical application.
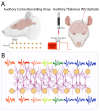
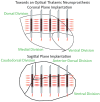
Aim: In this study, we assessed the local network dynamics of INS entrainment in the auditory thalamocortical circuit using the chronically implanted rat model; our approach focused on measuring INS energy-based local field potential (LFP) recruitment induced by focal thalamocortical stimulation. We further characterized linear and nonlinear oscillatory LFP activity in response to single-pulse and periodic INS and performed spectral decomposition to uncover specific LFP band entrainment to INS. Finally, we examined spike-field transformations across the thalamocortical synapse using spike-LFP coherence coupling.
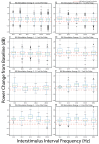
Results: We found that INS significantly increases LFP amplitude as a log-linear function of INS energy per pulse, primarily entraining to LFP and bands with synchrony extending to 200 Hz in some cases. A subset of neurons demonstrated nonlinear, chaotic oscillations linked to information transfer across cortical circuits. Finally, we utilized spike-field coherences to correlate spike coupling to LFP frequency band activity and suggest an energy-dependent model of network activation resulting from INS stimulation.
Conclusions: We show that INS reliably drives robust network activity and can potently modulate cortical field potentials across a wide range of frequencies in a stimulus parameter-dependent manner. Based on these results, we propose design principles for developing full coverage, all-optical thalamocortical auditory neuroprostheses.
Keywords: Chaos; Cochlear Implant; Cortex; Deep Brain Stimulation; Infrared Neural Stimulation; Thalamocortical Circuits; Thalamus.
Conflict of interest statement
Disclosures BSC is an unpaid scientific consultant for BECATech Inc for work unrelated to the present study. BSC also holds provisional patents in neural spectral decomposition methods. The other authors declare no conflicts of interest for the data presented in this study.
Figures





Similar articles
-
Aberrant thalamocortical coherence in an animal model of tinnitus.J Neurophysiol. 2019 Mar 1;121(3):893-907. doi: 10.1152/jn.00053.2018. Epub 2019 Jan 9. J Neurophysiol. 2019. PMID: 30625004 Free PMC article.
-
Spatially specific, closed-loop infrared thalamocortical deep brain stimulation.bioRxiv [Preprint]. 2023 Oct 19:2023.10.04.560859. doi: 10.1101/2023.10.04.560859. bioRxiv. 2023. Update in: PNAS Nexus. 2024 Feb 22;3(2):pgae082. doi: 10.1093/pnasnexus/pgae082. PMID: 37904955 Free PMC article. Updated. Preprint.
-
Focal Local Field Potential Signature of the Single-Axon Monosynaptic Thalamocortical Connection.J Neurosci. 2017 May 17;37(20):5123-5143. doi: 10.1523/JNEUROSCI.2715-16.2017. Epub 2017 Apr 21. J Neurosci. 2017. PMID: 28432143 Free PMC article.
-
Characterization and closed-loop control of infrared thalamocortical stimulation produces spatially constrained single-unit responses.PNAS Nexus. 2024 Feb 22;3(2):pgae082. doi: 10.1093/pnasnexus/pgae082. eCollection 2024 Feb. PNAS Nexus. 2024. PMID: 38725532 Free PMC article.
-
Phase correlation among rhythms present at different frequencies: spectral methods, application to microelectrode recordings from visual cortex and functional implications.Int J Psychophysiol. 1997 Jun;26(1-3):171-89. doi: 10.1016/s0167-8760(97)00763-0. Int J Psychophysiol. 1997. PMID: 9203002 Review.
References
-
- Cayce J. M. et al., “Infrared neural stimulation of thalamocortical brain slices,” IEEE Journal on Selected Topics in Quantum Electronics 16(3), 565–572 (2010) [doi:10.1109/JSTQE.2009.2032424]. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
