This is a preprint.
Environmental NaCl affects C. elegans development and aging
- PMID: 40161617
- PMCID: PMC11952357
- DOI: 10.1101/2025.03.09.641258
Environmental NaCl affects C. elegans development and aging
Update in
-
Environmental NaCl affects Caenorhabditis elegans development and aging.Genetics. 2025 Oct 8;231(2):iyaf139. doi: 10.1093/genetics/iyaf139. Genetics. 2025. PMID: 40820329
Abstract
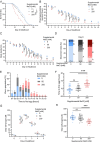
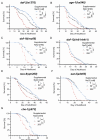
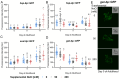
Sodium is an essential nutrient, but is toxic in excess. In humans, excessive dietary sodium can cause high blood pressure, which contributes to age-related diseases including stroke and heart disease. We used C. elegans to elucidate how sodium levels influence animal aging. Most experiments on this animal are conducted in standard culture conditions: Nematode Growth Medium (NGM) agar with a lawn of E. coli. Here, we report that the supplemental NaCl in standard NGM, 50 mM, accelerates aging and decreases lifespan. For comparison, we prepared NGM with reduced NaCl or excess NaCl. Considering reduced NaCl as a baseline, wild-type worms on standard NGM displayed normal development and fertility but reduced lifespan and health span, indicating toxicity in old animals. The long-lived mutants daf-2, age-1, and nuo-6, cultured on standard NGM, also displayed reduced lifespan. Thus, NaCl in standard NGM accelerates aging in multiple genetic backgrounds. Wild-type worms on excess NaCl displayed delayed development and reduced fertility, and reduced lifespan and health span, indicating toxicity in both young and old animals. These results suggest that young animals are relatively resistant to NaCl toxicity, but that aging causes progressive sensitivity, such that old animals display toxicity to both standard and excess NaCl. We investigated pathways that respond to NaCl. Young animals cultured with excess NaCl activated gpdh-1, a specific response to NaCl stress. Old animals cultured with excess NaCl activated gpdh-1 and hsp-6, a reporter for the mitochondrial unfolded protein response. Thus, excess NaCl activates multiple stress response pathways in older animals.
Keywords: C. elegans; aging; daf-2; development; lifespan; salt; sodium chloride (NaCl).
Figures

References
-
- Adachi H, Fujiwara Y, Ishii N. 1998. Effects of Oxygen on Protein Carbonyl and Aging in Caenorhabditis elegans Mutants With Long (age-1) and Short (mev-1) Life Spans. The Journals of Gerontology: Series A. 53A(4):B240–B244. doi: 10.1093/GERONA/53A.4.B240. [accessed 2024 Sep 19]. https://dx.doi.org/10.1093/gerona/53A.4.B240. - DOI - PubMed
-
- Anderson EN, Corkins ME, Li JC, Singh K, Parsons S, Tucey TM, Sorkaç A, Huang H, Dimitriadi M, Sinclair DA, et al. 2016. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech Ageing Dev. 154:30–42. doi: 10.1016/J.MAD.2016.01.004. - DOI - PMC - PubMed
-
- Bar-Ziv R, Bolas T, Dillin A. 2020. Systemic effects of mitochondrial stress. EMBO Rep. 21(6). doi: 10.15252/embr.202050094. [accessed 2020 Aug 30]. https://www.embopress.org/doi/10.15252/embr.202050094. - DOI - PMC - PubMed
-
- Bar-Ziv R, Frakes AE, Higuchi-Sanabria R, Bolas T, Frankino PA, Gildea HK, Metcalf MG, Dillin A. 2020. Measurements of physiological stress responses in C. Elegans . Journal of Visualized Experiments. 2020(159):1–21. doi: 10.3791/61001. [accessed 2021 Mar 4]. www.jove.com URL:https://www.jove.com/video/61001. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
