This is a preprint.
Intranasal AAV Vaccination of SARS-CoV-2 Induce Strong and Sustained Neutralizing Antibodies in Mice
- PMID: 40161689
- PMCID: PMC11952548
- DOI: 10.1101/2025.03.14.640671
Intranasal AAV Vaccination of SARS-CoV-2 Induce Strong and Sustained Neutralizing Antibodies in Mice
Abstract
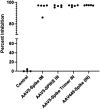
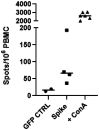
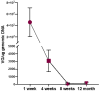
The COVID-19 pandemic continues to pose significant health challenges, despite existing vaccines. This study evaluates the immunogenicity of recombinant adeno-associated viruses (AAV) expressing SARS-CoV-2 spike proteins, administered intramuscularly and intranasally in mice. Both delivery methods of AAV5-spike, AAV5-spike stabilized trimer as well as AAV44.9-spike elicited robust serum anti-spike antibodies within 8-12 weeks, with high levels of anti-spike antibodies sustained for over a year. Comparison of mouse serum antibodies 16 weeks post intramuscular or intranasal AAV5 administration demonstrated similar SARS-CoV-2 spike binding neutralizing activity in vitro. Analysis of changes in cellular immunity by ELISpot at 12 weeks post-AAV spike transduction revealed interferon-γ induction in response to peptide challenge. Despite a decline in AAV vector DNA at the injection site, the persistence of anti-spike antibodies demonstrated that AAV-vectors can elicit lasting immune responses, highlighting nasal AAV administration as a potential strategy to block respiratory virus infections.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures

References
-
- Collier AY, Brown CM, McMahan KA, Yu J, Liu J, Jacob-Dolan C, et al. Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant. Sci Transl Med. 2022;14(641):eabn6150. Epub 20220420. doi: 10.1126/scitranslmed.abn6150. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
