This is a preprint.
Preventing neuropathy and improving anti-cancer chemotherapy with a carbazole-based compound
- PMID: 40161707
- PMCID: PMC11952460
- DOI: 10.1101/2025.03.10.642317
Preventing neuropathy and improving anti-cancer chemotherapy with a carbazole-based compound
Update in
-
Preventing neuropathy and improving anticancer chemotherapy with a carbazole-based compound.Sci Adv. 2025 Oct 31;11(44):eadw6328. doi: 10.1126/sciadv.adw6328. Epub 2025 Oct 29. Sci Adv. 2025. PMID: 41160692 Free PMC article.
Abstract
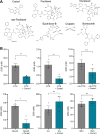
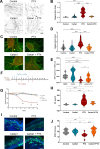
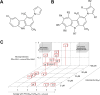
Advances in cancer treatment have led to a steady increase in the rate of disease remission. However, while many treatment-related adverse effects gradually resolve after therapy, chemotherapy-induced peripheral neuropathy (CIPN) often persists, with no means of prevention or direct treatment available. Herein, we present Carba1, a novel bi-functional carbazole that mitigates neuropathy through two distinct mechanisms. First, by interacting with tubulin, Carba1 reduces the required dose of taxanes, widely used chemotherapy drugs notorious for their toxic side effects, including CIPN. Second, Carba1 activates nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme in the NAD salvage pathway, triggering a metabolic rewiring that enhances the resilience of neurons and Schwann cells against chemotherapy-induced toxicity. We demonstrate the neuroprotective efficacy of Carba1 both in vitro, against neurotoxicity induced by paclitaxel (PTX), cisplatin, and bortezomib, and in vivo in a rat model of PTX-induced neuropathy. Importantly, we establish that Carba1 does not compromise the therapeutic efficacy of PTX nor promotes tumor growth. Comparative analyses of Carba1 derivatives further suggest the potential of designing compounds with either dual synergistic and neuroprotective activity or exclusive neuroprotective properties. Altogether, our findings position Carba1 as a promising therapeutic candidate for preventing CIPN, with the potential, if successfully translated to clinical settings, to improve both the quality of life and treatment outcome for cancer patients.
Conflict of interest statement
Competing interests: L.B. and L.L. are co-founders of Saxol SAS and co-inventors, with PD and PS, of the European patent # 24305705.6 “Carbazole derivatives used as neuroprotectants and in the treatment of disorders with reduced NAD metabolism”. The other authors declare that they have no competing interests in relation to the work described.
Figures




References
-
- Walsh V., Goodman J., From taxol to taxol®: The changing identities and ownership of an anti-cancer drug. Med. Anthropol. 21, 307–336 (2002). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
