This is a preprint.
Antibody-Free Immunopeptide Nano-Conjugates for Brain-Targeted Drug Delivery in Glioblastoma Multiforme
- PMID: 40161747
- PMCID: PMC11952356
- DOI: 10.1101/2025.03.07.641755
Antibody-Free Immunopeptide Nano-Conjugates for Brain-Targeted Drug Delivery in Glioblastoma Multiforme
Update in
-
Antibody-Free Immunopeptide Nanoconjugates for Brain-Targeted Drug Delivery in Glioblastoma Multiforme.Bioconjug Chem. 2025 Oct 15;36(10):2132-2144. doi: 10.1021/acs.bioconjchem.5c00168. Epub 2025 Jul 2. Bioconjug Chem. 2025. PMID: 40601862
Abstract
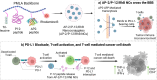
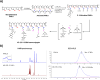
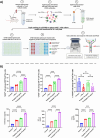
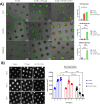
Glioblastoma Multiforme (GBM) represents a significant clinical challenge amongst central nervous system (CNS) tumors, with a dismal mean survival rate of less than 8 months, a statistic that has remained largely unchanged for decades (National Brain Society, 2022). The specialized intricate anatomical features of the brain, notably the blood-brain barrier (BBB), pose significant challenges to effective therapeutic interventions, limiting the potential reach of modern advancements in immunotherapy to impact these types of tumors. This study introduces an innovative, actively targeted immunotherapeutic nanoconjugate (P12/AP-2/NCs) designed to serve as an immunotherapeutic agent capable of traversing the BBB via LRP-1 receptor-mediated transcytosis. P12/AP-2/NCs exert its immune-modulating effects by inhibiting the PD-1/PD-L1 axis through a small-size PD-L1/PD-L2 antagonist peptide Aurigene NP-12 (P12). P12/AP-2/NCs are synthesized from completely biodegradable, functionalized high molecular weight β-poly(L-malic acid) (PMLA) polymer, conjugated with P12 and Angiopep-2 (AP2) to yield P12/AP-2/NCs. Evaluating nanoconjugates for BBB permeability and 3-D tumor model efficacy using an in vitro BBB-Transwell spheroid based model demonstrating successful crossing of the BBB and internalization in brain 3D tumor environments. In addition, the nanoconjugate mediated T cell's cytotoxicity on 3D tumor region death in a U87 GBM 3-D spheroid model. AP2/P12/NCs is selectively inhibited in PD1/PDL1 interaction on T cells and tumor site, increasing inflammatory cytokine secretion and T cell proliferation. In an in-vivo murine brain environment, rhodamine fluorophore-labeled AP2/P12/NCs displayed significantly increased accumulation in the brain during 2-6 h time intervals post-injection with a prolonged bioavailability over unconjugated peptides. AP2/P12/NCs demonstrated a safety profile at both low and high doses based on major organ histopathology evaluations. Our findings introduce a novel, programmable nanoconjugate platform capable of penetrating the BBB for directed delivery of small peptides and significant immune environment modulation without utilizing antibodies, offering promise for treating challenging brain diseases like glioblastoma multiforme and beyond.
Keywords: 3D Tumor-BBB Model; Biodistribution; Immunotherapy; Nanoconjugate.
Figures


References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
