This is a preprint.
IL-6 underlies microenvironment immunosuppression and resistance to therapy in glioblastoma
- PMID: 40161763
- PMCID: PMC11952432
- DOI: 10.1101/2025.03.12.642800
IL-6 underlies microenvironment immunosuppression and resistance to therapy in glioblastoma
Abstract
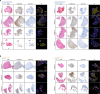
The glioblastoma tumor immune microenvironment (TIME) is an immunosuppressive barrier to therapy that encumbers glioblastoma responses to immune checkpoint inhibition (ICI). Immunosuppressive cytokines, pro-tumor myeloid cells, and exhausted T-cells are hallmarks of the glioblastoma TIME. Here we integrate spatial and single-cell analyses of patient-matched human glioblastoma samples before and after ICI with genetic, immunologic, single-cell, and pharmacologic studies in preclinical models to reveal that interleukin-6 (IL-6) inhibition reprograms the glioblastoma TIME to sensitize mouse glioblastoma to ICI and radiotherapy. Rare human glioblastoma patients who achieve clinical responses to ICI have lower pre-treatment IL-6 levels compared to glioblastomas who do not respond to ICI. Immune stimulatory gene therapy suppresses IL-6 tumor levels in preclinical murine models of glioblastoma. Furthermore, survival was longer in Il-6 knockout mice with orthotopic SB28 glioblastoma relative to wild-type mice. IL-6 blockade with a neutralizing antibody transiently sensitizes mouse glioblastoma to anti-PD-1 by increasing MHCII+ monocytes, CD103+ migratory dendritic cells (DCs), CD11b+ conventional DCs, and effector CD8+ T cells, and decreasing immunosuppressive Tregs. To translate these findings to a combination treatment strategy for recurrent glioblastoma patients, we show that IL-6 blockade plus ICI durably sensitizes mouse glioblastoma to high-dose radiotherapy.
Conflict of interest statement
Competing interests statement The authors declare no competing interests.
Figures




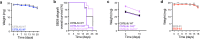















References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
