This is a preprint.
Microtubule-dependent cell polarity regulates skin-resident macrophage phagocytosis and directed cell migration
- PMID: 40161838
- PMCID: PMC11952491
- DOI: 10.1101/2025.03.13.642867
Microtubule-dependent cell polarity regulates skin-resident macrophage phagocytosis and directed cell migration
Abstract
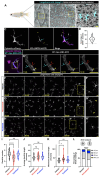
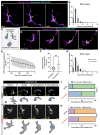
Immune cells rapidly respond to tissue damage through dynamic properties of the cytoskeleton. How microtubules control immune cell functions during injury responses remains poorly understood. Within skin, tissue-resident macrophages known as Langerhans cells use dynamic dendrites to surveil the epidermis for damage and migrate through a densely packed epithelium to wounds. Here, we use Langerhans cells within the adult zebrafish epidermis as a model to investigate roles for microtubules in immune cell tissue surveillance, phagocytosis, and directed migration. We describe microtubule organization within Langerhans cells, and show that depolymerizing the microtubule cytoskeleton alters dendrite morphology, debris engulfment, and migration efficiency. We find that the microtubule organizing center positions adjacent to engulfed debris and that its position correlates with navigational pathfinding during directed cell migration. Stabilizing microtubules prevents Langerhans cell motility during directed cell migration by impairing navigation around cellular obstacles. Collectively, our work demonstrates requirements for microtubules in the dynamic actions of tissue-resident macrophages during epithelial surveillance and wound repair.
Keywords: Microtubule; migration; phagocytosis; skin; tissue-resident macrophage; zebrafish.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Rho-associated kinase regulates Langerhans cell morphology and responsiveness to tissue damage.bioRxiv [Preprint]. 2023 Jul 29:2023.07.28.550974. doi: 10.1101/2023.07.28.550974. bioRxiv. 2023. Update in: Cell Rep. 2024 May 28;43(5):114208. doi: 10.1016/j.celrep.2024.114208. PMID: 37546841 Free PMC article. Updated. Preprint.
-
Rho-associated kinase regulates Langerhans cell morphology and responsiveness to tissue damage.Cell Rep. 2024 May 28;43(5):114208. doi: 10.1016/j.celrep.2024.114208. Epub 2024 May 9. Cell Rep. 2024. PMID: 38728139
-
Calcium dynamics of skin-resident macrophages during homeostasis and tissue injury.bioRxiv [Preprint]. 2024 Sep 26:2024.09.24.614510. doi: 10.1101/2024.09.24.614510. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 Dec 1;35(12):br26. doi: 10.1091/mbc.E24-09-0420. PMID: 39386455 Free PMC article. Updated. Preprint.
-
Cytoskeleton network participates in the anti-infection responses of macrophage.Bioessays. 2023 Aug;45(8):e2200225. doi: 10.1002/bies.202200225. Epub 2023 May 31. Bioessays. 2023. PMID: 37254735 Review.
-
Regulation of Cell Adhesion and Migration via Microtubule Cytoskeleton Organization, Cell Polarity, and Phosphoinositide Signaling.Biomolecules. 2023 Sep 22;13(10):1430. doi: 10.3390/biom13101430. Biomolecules. 2023. PMID: 37892112 Free PMC article. Review.
References
-
- Alraies Z., Rivera C.A., Delgado M.-G., Sanséau D., Maurin M., Amadio R., Maria Piperno G., Dunsmore G., Yatim A., Lacerda Mariano L., Kniazeva A., Calmettes V., Sáez P.J., Williart A., Popard H., Gratia M., Lamiable O., Moreau A., Fusilier Z., Crestey L., Albaud B., Legoix P., Dejean A.S., Le Dorze A.-L., Nakano H., Cook D.N., Lawrence T., Manel N., Benvenuti F., Ginhoux F., Moreau H.D., Nader G. P. F., Piel M., and Lennon-Duménil A.-M.. 2024. Cell shape sensing licenses dendritic cells for homeostatic migration to lymph nodes. Nat Immunol. 25:1193–1206. doi:10.1038/s41590-024-01856-3. - DOI - PMC - PubMed
-
- Athlin L., Domellof L., and Norberg B.O.. 1986. The phagocytosis of yeast cells by monocytes: effects of colchicine, beta-lumicolchicine, and gamma-lumicolchicine. Lymphology. 19:130–135. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
