Navigating mesenchymal stem cells doses and delivery routes in heart disease trials: A comprehensive overview
- PMID: 40162019
- PMCID: PMC11952810
- DOI: 10.1016/j.reth.2025.02.012
Navigating mesenchymal stem cells doses and delivery routes in heart disease trials: A comprehensive overview
Abstract
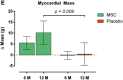
In recent years, various clinical trials have been designed and implemented using mesenchymal stem cells (MSCs) for the treatment of heart diseases. Clinical trials exploring MSC-based treatments have proliferated, yet the lack of standardized protocols for MSC administration remains a significant challenge. Despite the growing popularity of MSC trials, questions persist regarding optimal dosing, administration routes, and frequency to achieve safety and efficacy, particularly in the context of cardiac regeneration. The current study has reviewed the clinical trials that have used MSCs for the treatment of heart diseases since 2009. The findings reveal diverse transplantation methods and varying MSCs quantities, highlighting the absence of a universal guideline for MSCs utilization in heart disease clinical trials.
Keywords: Administration routes; Clinical trial; Heart diseases; Mesenchymal stem cells; Optimal doses.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies.Tissue Eng Part B Rev. 2020 Dec;26(6):555-570. doi: 10.1089/ten.TEB.2019.0351. Epub 2020 May 20. Tissue Eng Part B Rev. 2020. PMID: 32242479 Review.
-
Mechanism of Action of Mesenchymal Stem Cells (MSCs): impact of delivery method.Expert Opin Biol Ther. 2022 Apr;22(4):449-463. doi: 10.1080/14712598.2022.2016695. Epub 2021 Dec 27. Expert Opin Biol Ther. 2022. PMID: 34882517 Free PMC article.
-
Mesenchymal Stromal Cells to Regenerate Emphysema: On the Horizon?Respiration. 2018;96(2):148-158. doi: 10.1159/000488149. Epub 2018 May 2. Respiration. 2018. PMID: 29719298 Free PMC article. Review.
-
Optimal mesenchymal stem cell delivery routes to enhance neurogenesis for the treatment of Alzheimer's disease: optimal MSCs delivery routes for the treatment of AD.Histol Histopathol. 2018 Jun;33(6):533-541. doi: 10.14670/HH-11-950. Epub 2017 Nov 29. Histol Histopathol. 2018. PMID: 29185257 Review.
-
Mesenchymal stem cell therapy in veterinary ophthalmology: clinical evidence and prospects.Vet Res Commun. 2024 Dec;48(6):3517-3531. doi: 10.1007/s11259-024-10522-w. Epub 2024 Aug 30. Vet Res Commun. 2024. PMID: 39212813 Review.
References
-
- Chow C.M., Donovan L., Manuel D., Johansen H., Tu J.V. Regional variation in self-reported heart disease prevalence in Canada. Can J Cardiol. 2005;21(14):1265–1271. - PubMed
-
- Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. - PubMed
-
- Ohnishi S., Ohgushi H., Kitamura S., Nagaya N. Mesenchymal stem cells for the treatment of heart failure. Int J Hematol. 2007;86:17–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

