This is a preprint.
At-Home Movement State Classification Using Totally Implantable Bidirectional Cortical-Basal Ganglia Neural Interface
- PMID: 40162212
- PMCID: PMC11952646
- DOI: 10.21203/rs.3.rs-6058394/v1
At-Home Movement State Classification Using Totally Implantable Bidirectional Cortical-Basal Ganglia Neural Interface
Abstract
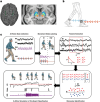
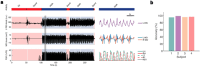
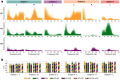
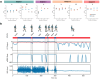
Movement decoding from invasive human recordings typically relies on a distributed system employing advanced machine learning algorithms programmed into an external computer for state classification. These brain-computer interfaces are limited to short-term studies in laboratory settings that may not reflect behavior and neural states in the real world. The development of implantable devices with sensing capabilities is revolutionizing the study and treatment of brain circuits. However, it is unknown whether these devices can decode natural movement state from recorded neural activity or accurately classify states in real-time using on-board algorithms. Here, using a totally implanted sensing-enabled neurostimulator to perform long-term, at-home recordings from the motor cortex and pallidum of four subjects with Parkinson's disease, we successfully identified highly sensitive and specific personalized signatures of gait state, as determined by wearable sensors. Additionally, we demonstrated the feasibility of using at-home data to generate biomarkers compatible with the classifier embedded on-board the neurostimulator. These findings offer a pipeline for ecologically valid movement biomarker identification that can advance therapy across a variety of diseases.
Keywords: Parkinson’s disease; deep brain stimulation; gait; globus pallidus; motor cortex; neurophysiology.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures


Similar articles
-
Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson's disease.J Neurosurg. 2018 Feb;128(2):605-616. doi: 10.3171/2016.11.JNS161162. Epub 2017 Apr 14. J Neurosurg. 2018. PMID: 28409730 Free PMC article.
-
Movement decoding using neural synchronization and inter-hemispheric connectivity from deep brain local field potentials.J Neural Eng. 2015 Oct;12(5):056011. doi: 10.1088/1741-2560/12/5/056011. Epub 2015 Aug 25. J Neural Eng. 2015. PMID: 26305124
-
Pallidal Deep-Brain Stimulation Disrupts Pallidal Beta Oscillations and Coherence with Primary Motor Cortex in Parkinson's Disease.J Neurosci. 2018 May 9;38(19):4556-4568. doi: 10.1523/JNEUROSCI.0431-18.2018. Epub 2018 Apr 16. J Neurosci. 2018. PMID: 29661966 Free PMC article.
-
Machine learning based brain signal decoding for intelligent adaptive deep brain stimulation.Exp Neurol. 2022 May;351:113993. doi: 10.1016/j.expneurol.2022.113993. Epub 2022 Jan 29. Exp Neurol. 2022. PMID: 35104499 Free PMC article. Review.
-
The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease.Brain. 1994 Aug;117 ( Pt 4):877-97. doi: 10.1093/brain/117.4.877. Brain. 1994. PMID: 7922472 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

