Comparative analysis of oral microbiome in molar-incisor-hypomineralization vs healthy age-matched controls
- PMID: 40162761
- PMCID: PMC12054143
- DOI: 10.1128/spectrum.02897-24
Comparative analysis of oral microbiome in molar-incisor-hypomineralization vs healthy age-matched controls
Abstract
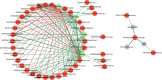
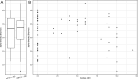
Molar-incisor-hypomineralization (MIH) is one of the most challenging dental diseases in children. While the association of oral microbiomes with caries and periodontitis has been studied thoroughly, limited data on the microbial composition in MIH and its clinical significance exist. This cross-sectional study aimed to compare the supragingival plaque microbiome between children and adolescents affected by MIH and a healthy age-matched control group. Ninety-five patients aged 7-17 years were recruited at the Department of Conservative Dentistry, Heidelberg University Hospital. The final sample included 29 participants with a confirmed diagnosis of MIH, treated preventively and restoratively, and 35 orally healthy controls. Clinical data were obtained, and supragingival plaque samples were collected using OMNIgene ORAL OMR-110 (DNA Genotek Inc.), followed by 16S rRNA amplicon sequencing. The microbiome composition was analyzed using α-diversity (Shannon index) and evenness (Pielou index), with group differences assessed using permutational multivariate analysis of variance (PERMANOVA) and MaAsLin2. The overall microbiome composition showed mostly similarities between both groups (PERMANOVA: R² = 0.019, P-value = 0.287), indicating no major dysbiosis. However, a significant decrease in α-diversity and evenness was observed with an increasing number of MIH-affected teeth. Pronounced positive correlations were found between ASV0055 (Streptococcus spp.), caries experience, and MIH severity. ASV0100 (Mannheimia sp.) increased significantly with the increasing number of MIH-affected teeth, whereas ASV0053 (Bergeyella sp.) decreased with higher caries experience. In summary, the oral microbiome of children and adolescents with MIH exhibits no significant differences from healthy children and adolescents of the same age group. Depending on MIH severity, the presence of early plaque-forming species and cariogenic biofilm may increase, requiring intensive, tailored preventive care and appropriate restorative treatment to achieve microbial homeostasis.
Importance: Molar-incisor-hypomineralization (MIH) represents a significant burden for affected children and adolescents, playing an increasingly important role in pediatric dentistry worldwide. Despite its high global prevalence, data on the microbiome of MIH patients remains limited. This study is the first to compare the oral microbiome composition of MIH patients with a healthy control group, making a significant contribution to pediatric dentistry and microbiology. Our results indicate that the oral microbiome of children with MIH is similar to that of healthy children of the same age. Although this structural anomaly predisposes patients to caries, effective preventive and restorative treatments can help maintain microbial homeostasis. However, MIH-affected children remain high-risk patients, as the disease severity may reduce microbial diversity. Furthermore, the increased abundance of Streptococcus spp. in MIH patients indicates a higher caries susceptibility, emphasizing the need for targeted dental care focusing on plaque control and topical fluoride use.
Keywords: Streptococcus; dental plaque; human microbiome; oral microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

