Combination of Glucagon-Like Peptide 1 Receptor Agonist and Thiazolidinedione for Mortality and Cardiovascular Outcomes in Patients With Type 2 Diabetes
- PMID: 40163115
- PMCID: PMC11959443
- DOI: 10.1001/jamanetworkopen.2025.2577
Combination of Glucagon-Like Peptide 1 Receptor Agonist and Thiazolidinedione for Mortality and Cardiovascular Outcomes in Patients With Type 2 Diabetes
Erratum in
-
Error in Affiliations.JAMA Netw Open. 2025 Jun 2;8(6):e2519672. doi: 10.1001/jamanetworkopen.2025.19672. JAMA Netw Open. 2025. PMID: 40459899 Free PMC article. No abstract available.
Abstract
Importance: Combination therapy has emerged as a critical area of interest in managing diabetes; however, the association of combination therapy with a glucagon-like peptide 1 receptor agonist (GLP-1RA) and thiazolidinedione and the risk of diabetes-related complications remains incompletely understood.
Objective: To compare the hazards of cardiovascular-related morbidities and mortality among patients with type 2 diabetes receiving combination therapy with a GLP-1RA plus thiazolidinedione, those receiving monotherapy with a GLP-1RA or thiazolidinedione, and nonusers.
Design, setting, and participants: This retrospective cohort study used nationwide data obtained from Taiwan's National Health Insurance Research Database. Patients older than 20 years with type 2 diabetes who received a GLP-1RA or thiazolidinedione between January 1, 2011, and December 31, 2020, were enrolled. The data analysis was performed from May 1 to May 22, 2024.
Main outcomes and measures: This study investigated the hazards of all-cause mortality, major adverse cardiovascular events, cardiovascular mortality, cardiovascular complications, and hypoglycemia in GLP-1RA and thiazolidinedione combination or monotherapy users compared with nonusers.
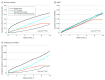
Results: A total of 110 411 patients were enrolled (mean [SD] age, 58.3 [11.9] years; 45.5% female; 47 526 GLP-1RA users, 32 203 thiazolidinedione users, and 30 682 GLP-1RA plus thiazolidinedione users), along with a propensity score-matched group of patients who did not use a GLP-1RA or thiazolidinedione, for a total cohort size of 220 822. Patients receiving GLP-1RA and thiazolidinedione dual therapy had significantly lower risk of all-cause mortality (adjusted hazard ratio [AHR], 0.20; 95% CI, 0.19-0.21; P < .001), major adverse cardiovascular events (AHR, 0.85; 95% CI, 0.82-0.89; P < .001), and cardiovascular mortality (AHR, 0.20; 95% CI, 0.18-0.23; P < .001) than those who did not receive a GLP-1RA or thiazolidinedione. However, a higher risk of hypoglycemia was seen in those receiving combination therapy (AHR, 1.61; 95% CI, 1.43-1.82; P < .001) and those receiving thiazolidinedione monotherapy (AHR, 1.69; 95% CI, 1.51-1.90; P < .001) compared with nonuse. This risk was mitigated with prolonged use. Thiazolidinedione monotherapy users had a significantly higher risk of all-cause mortality (AHR, 1.29; 95% CI, 1.24-1.34; P < .001) and cardiovascular mortality (AHR, 1.28; 95% CI, 1.13-1.45; P < .001) than GLP-1RA monotherapy users. Several sensitivity analyses further supported the robustness of these findings.
Conclusions and relevance: In this cohort study of patients with type 2 diabetes, combination therapy with a GLP-1RA plus thiazolidinedione was associated with significantly lower hazards of mortality and cardiovascular complications compared with nonuse. The findings suggest that GLP-1RAs may mitigate the adverse cardiovascular effects of thiazolidinedione.
Conflict of interest statement
Figures


References
-
- Saxena AR, Frias JP, Brown LS, et al. Efficacy and safety of oral small molecule glucagon-like peptide 1 receptor agonist danuglipron for glycemic control among patients with type 2 diabetes: a randomized clinical trial. JAMA Netw Open. 2023;6(5):e2314493. doi: 10.1001/jamanetworkopen.2023.14493 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

