N-Acetylcysteine for Hereditary Cystatin C Amyloid Angiopathy: A Nonrandomized Clinical Trial
- PMID: 40163249
- PMCID: PMC11959480
- DOI: 10.1001/jamaneurol.2025.0326
N-Acetylcysteine for Hereditary Cystatin C Amyloid Angiopathy: A Nonrandomized Clinical Trial
Abstract
Importance: Hereditary cystatin C amyloid angiopathy (HCCAA) is a lethal, dominantly inherited disease primarily affecting Icelandic young adults that leads to severe cerebral amyloid angiopathy, with no effective therapy.
Objective: To investigate safety, tolerance, and therapeutic potential of N-acetylcysteine (NAC) in lowering disease-associated biomarkers in sequence variation carriers.
Design, setting, and participants: This phase 2a open-label clinical trial was conducted from March 2019 to December 2021 at a single study center at Landspitali University Hospital in Reykjavik, Iceland, and included 17 confirmed carriers of the L68Q-CST3 sequence variation.
Intervention: High-dose NAC treatment was administered at 2400 mg daily for 9 months. Participants underwent regular monitoring for hemorrhages and disease progression, including blood and skin biopsy samples obtained every 3 months for biomarker testing.
Main outcomes and measures: The primary outcomes were drug tolerability and safety, cognitive status, and reduction in disease-associated biomarkers in skin biopsies. Secondary outcomes included changes in blood and plasma biomarker levels.
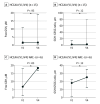
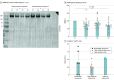
Results: Of 17 carriers treated, 6 were male and 11 were female, and mean (SD) participant age was 40.0 (4.2) years. Analysis of the primary outcomes showed that NAC was safe and well tolerated. Five cerebral bleeds occurred during the treatment period without permanent neurological sequela; no death occurred. There was significant reduction in median (IQR) disease-specific biomarker levels in skin after treatment, including collagen IV (baseline: 3.69% [2.48%-5.16%]; after treatment: 2.60% [1.99%-2.97%]; P < .001), fibronectin (baseline: 3.17% [2.09%-5.05%]; after treatment: 2.37% [1.87%-3.42%]; P = .01), vimentin (baseline: 1.60% [1.24%-2.37%]; after treatment: 1.31% [0.97%-1.68%]; P < .001), and SMAD (baseline: 2.25% [0.55%-4.36%]; after treatment: 1.56% [0.20%-2.54%]; P < .001) via Wilcoxon matched-pairs signed rank test. Secondary outcomes included a significant increase in reduced glutathione levels and decreased high-molecular-weight cystatin C aggregate levels in plasma after NAC treatment.
Conclusions and relevance: In this single-center nonrandomized clinical trial, NAC was safe and well tolerated and decreased disease-associated biomarker and amyloid deposition, suggesting NAC may offer a preventive strategy against HCCAA.
Trial registration: ClinicalTrialsRegister.eu Identifier: 2017-004776-56.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

