Exposure to circadian disrupting environment and high-fat diet during pregnancy and lactation alter reproductive competence and lipid profiles of liver, mammary, plasma and milk of ICR mice
- PMID: 40163509
- PMCID: PMC11957368
- DOI: 10.1371/journal.pone.0320538
Exposure to circadian disrupting environment and high-fat diet during pregnancy and lactation alter reproductive competence and lipid profiles of liver, mammary, plasma and milk of ICR mice
Abstract
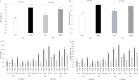
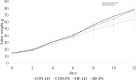
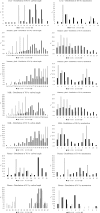
This study's objective was to determine the effects of pre-pregnancy obesity induced by a high-fat diet and exposure to circadian-disrupting light-dark phase shifts on birth littler size, pup survival to 24h and growth to lactation day 12, and their relationship to maternal feeding patterns, fecal corticosterone levels, milk composition, and lipid profiles of liver, plasma, mammary gland, and milk. A 2 by 2 factorial designed experiment of female ICR mice assigned to control (CON; 10% fat) or high-fat (HF; 60% fat) and either a 12-hour light-dark (LD) cycle or a chronic jet lag model of 6-hour phase-shifts (PS) in light-dark cycle every 3 days throughout pregnancy and lactation, resulted in 4 treatment groups: CON-LD, CON-PS, HF-LD and HF-PS. HF diet increased maternal pre-pregnancy body weight and elevated milk lactose. Whereas PS reduced milk lactose within the CON diet group, and increased maternal feed intake and fecal corticosterone levels. PS exposure also affected the time of day of birth. Neither PS nor HF affected birth litter size or pup survival. Only diet impacted final litter weight, with HF greater than CON. Among the 1204 lipids detected by multiple reaction monitoring (MRM)-profiling, diet altered 67.1% in milk, 58.1% in mammary gland, 27.2% in the liver, and 10.9% in plasma, with HF increasing the carbon length of diacylglycerols in the liver and milk, and carbon length of triacylglycerols in plasma, mammary gland and milk. Although exposure to PS had no overall impact on maternal lipid profiles, interactions (P < 0.05) were found between PS and diets in the phosphatidylcholine and phosphatidylethanolanine class of lipids. Findings support that high fat diet and exposure to circadian disrupting environments impact maternal feeding behavior and stress responses as well as lipid profiles, which may relate to their negative association with maternal health and offspring development.
Copyright: © 2025 Reis et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, et al.. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607–608:1073–84. doi: 10.1016/j.scitotenv.2017.07.056 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

