Safety and effectiveness of the first balloon-in-basket pulsed field ablation system for the treatment of atrial fibrillation: VOLT CE Mark Study 6-month results
- PMID: 40163671
- PMCID: PMC12036658
- DOI: 10.1093/europace/euaf072
Safety and effectiveness of the first balloon-in-basket pulsed field ablation system for the treatment of atrial fibrillation: VOLT CE Mark Study 6-month results
Abstract
Aims: Pulsed field ablation (PFA) is a growing ablation modality for pulmonary vein isolation (PVI) in atrial fibrillation (AF) patients. This study assesses the 6-month safety and effectiveness of a novel balloon-in-basket, mapping-integrated PFA system, with a purpose-built form factor for PVI.
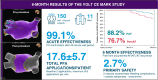
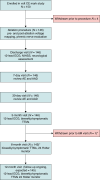
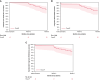
Methods and results: The VOLT CE Mark Study is a prospective, multi-center, pre-market study. A total of 150 patients with drug-refractory paroxysmal (PAF) or persistent AF (PersAF) were enrolled between 8 November 2023 and 14 March 2024, of which 146 patients (age 64.1 ± 10.0 years, 63.0% male, 70.5% PAF) underwent PVI with the balloon-in-basket PFA catheter and system featuring integrated electroanatomic mapping with contact-sensing. Study endpoints were the rate of primary serious adverse events within 7 days as well as acute procedural effectiveness and 6-month freedom from recurrence. Acute effectiveness was achieved in 99.1% (573/578) of treated PVs (98.6% of patients, 144/146) with 17.6 ± 5.7 PFA applications/patient. Procedure, fluoroscopy, LA dwell, and transpired ablation times were 100.4 ± 33.0, 17.3 ± 12.1, 39.4 ± 20.6, and 31.4 ± 16.8 min, respectively. There were 4 (2.7%; 4/146) primary serious adverse events. The rate of freedom from documented atrial arrhythmias was 88.2% in PAF patients and 76.7% in PersAF patients (freedom from symptomatic recurrence was documented in 90.2% of PAF patients and 74.4% of PersAF patients) through 6-months post-index procedure.
Conclusion: The VOLT CE Mark Study primary results demonstrate the safety and effectiveness of the novel balloon-in-basket PFA system to perform PVI in PAF and PersAF.
Keywords: Atrial fibrillation; Balloon; Balloon-in-basket; Basket; Catheter ablation; Pulmonary vein isolation; Pulsed field ablation; Single-shot.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.R.T. is a consultant for Boston Scientific, Philiips, Medtronic Biosense Webster, Abbott Medical, he received speaker`s honoraria from Boston Scientific, Biotronik, Biosense Webster, Abbott Medical, Lifetech, Pfizer and research grants from Abbott, Biotronik, Medtronic, Biosense Webster, Lifetech. He received travel grants from Abbott, Biosense Webster, Boston Scientific, Medtronic and Philips. G.B.C. received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Boston Scientific, and Acutus Medical. M.G. reports speaker‘s honoraria, travel grants, or consulting honoraria for Abbott, Biosense Webster, Boston Scientific, Medtronic, Farapulse Inc, Emmar, Lumavision, Biotronik, Bristol-Myers Squibb, Zoll. M.G. has served on advisory boards for Boston Scientific, Medtronic, and Abbott. P.S. reports having served on the advisory board of Boston Scientific, CathRx, Medtronic, Abbott Medical, and PaceMate. P.S. reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Boston Scientific, Abbott Medical, PaceMate, and CathRx. P.S. reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott Medical, Boston Scientific, PaceMate, and Becton Dickinson. H.H. has received speaking honoraria and consulting fees from Boston Scientific, Medtronic, and Abbott. J.K. reports research and fellowship support from Medtronic, Biosense Webster, Abbott, and Zoll. S.H. reports teaching and consulting honorariums for Medtronic, Boston Scientific, Biotronik, and Johnson and Johnson. S.H. also serves on the medical advisory boards for Biotronik and Boston Scientific. The Victorian Heart Hospital has also received research funds from Boston Scientific, Medtronic, and Abbott on behalf of S.H. The University of Adelaide has received advisory and/or consulting fees on behalf of M.E. from Medtronic and Biosense Webster. H.P. reports consulting fees/honoraria from Abbott, Biotronik, Boston Scientific, Biosense Webster and Medtronic. V.Y.R. is a consultant to and has received equity from Affera-Medtronic; and unrelated to this manuscript, V.Y.R. has served as a consultant for and has equity in Ablacon, Acutus Medical, Anumana, Apama Medical-Boston Scientific, APN Health, Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, Cardiofocus, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Farapulse-Boston Scientific, Field Medical, Focused Therapeutics, HRT, Intershunt, Javelin, Kardium, Keystone Heart, Laminar Medical, LuxMed, Medlumics, Middlepeak, Neutrace, Nuvera-Biosense Webster, Oracle Health, Restore Medical, Sirona Medical, SoundCath, and Valcare; unrelated to this work, V.Y.R. has served as a consultant for Abbott, Adagio Medical, Append Medical, AtriAN, Biosense-Webster, BioTel Heart, Biotronik, Boston Scientific, Cairdac, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Novo Nordisk, Philips, and Pulse Biosciences; and unrelated to this work, V.Y.R. has equity in Atraverse, DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Soundcath, Surecor, and Vizaramed. E.J., N.D., A.M., and D.W. are employed by Abbott. D.L. is a consultant for Abbott, Biotronic, Biosense Webster, Medtronic, Boston Scientific, AtriCure, Acutus, and Northeast Scientific. The remaining authors have no further disclosures.
Figures



Comment in
-
A new basket player on the court: pulsed field ablation with a balloon-in-basket system for atrial fibrillation.Europace. 2025 Mar 28;27(4):euaf065. doi: 10.1093/europace/euaf065. Europace. 2025. PMID: 40163667 Free PMC article. No abstract available.
References
-
- Tzeis S, Gerstenfeld EP, Kalman J, Saad EB, Sepehri Shamloo A, Andrade JG et al. 2024 European heart rhythm association/heart rhythm society/Asia Pacific heart rhythm society/Latin American heart rhythm society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024;26:1–107.
-
- Leitz P, Mönnig G, Güner F, Dechering DG, Wasmer K, Reinke F et al. Comparing learning curves of two established “single-shot” devices for ablation of atrial fibrillation. J Interv Card Electrophysiol 2018;53:317–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

