AhR activation mitigates graft-versus-host disease of the central nervous system by reducing microglial NF-κB signaling
- PMID: 40163754
- PMCID: PMC12182783
- DOI: 10.1182/bloodadvances.2024015000
AhR activation mitigates graft-versus-host disease of the central nervous system by reducing microglial NF-κB signaling
Abstract
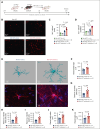
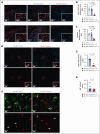
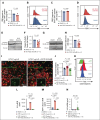
Acute graft-versus-host disease (GVHD) that occurs after allogeneic hematopoietic cell transplantation (allo-HCT) can affect the central nervous system (CNS). Most patients who have undergone allo-HCT receive antibiotic treatment, which alters the microbiome and essential microbiome-derived metabolites. We investigated the impact of microbiome modifications on CNS GVHD and therapeutic strategies to overcome the microbiome-derived metabolite depletion. Antibiotic treatment of mice undergoing allo-HCT increased microglia numbers in the brain, indicating increased inflammation. In addition, microglial morphology shifted toward a highly branched phenotype. Consistent with a proinflammatory phenotype, the microglia exhibited increased NF-κB and Src activity. Antibiotic treatment caused the depletion of the bacteria-derived aryl hydrocarbon receptor (AhR) ligand indole-3-acetate in the brain. Conversely, treatment of the primary microglia with the AhR ligand 6-formylindolo(3,2-b)carbazole (FICZ) reduced NF-κB activity and phagocytic potential. Microglia expansion and morphological changes were reversed by AhR ligand FICZ treatment. Moreover, the AhR ligand indole-3-acetate was also reduced in the CNS of patients who developed acute GVHD concomitant with increased microglial NF-κB expression. In summary, we demonstrated that antibiotic treatment and a subsequent decrease of AhR ligands resulted in increased microglial activation in CNS GVHD. FICZ treatment hampered CNS inflammation by inhibiting NF-κB activity, thereby providing a metabolic modifier to interfere with pathogenic microglia signaling and CNS GVHD in vivo.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.Z. has received honoraria from Novartis, Incyte, Sanofi, and Mallinckrodt. D.M. received research grants from Novartis and Sanofi, and consulting fees from Novartis, Incyte, Sanofi, CSL Behring, Jazz Pharmaceuticals, and Mallinckrodt. The remaining authors declare no competing financial interests.
Figures






References
-
- Cusatis R, Litovich C, Feng Z, et al. Current trends and outcomes in cellular therapy activity in the United States, including prospective patient-reported outcomes data collection in the Center for International Blood and Marrow Transplant Research registry. Transpl Cell Ther. 2024;30(9):917.e1–917.e12. - PMC - PubMed
-
- Zeiser R, Teshima T. Nonclassical manifestations of acute GVHD. Blood. 2021;138(22):2165–2172. - PubMed
-
- Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52(5):460–470. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

