Treat-to-target of endoscopic remission in patients with inflammatory bowel disease in symptomatic remission on advanced therapies (QUOTIENT): rationale, design and protocol for an open-label, multicentre, pragmatic, randomised controlled trial
- PMID: 40164445
- PMCID: PMC11962770
- DOI: 10.1136/bmjgast-2024-001615
Treat-to-target of endoscopic remission in patients with inflammatory bowel disease in symptomatic remission on advanced therapies (QUOTIENT): rationale, design and protocol for an open-label, multicentre, pragmatic, randomised controlled trial
Abstract
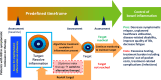
Introduction: Targeted immunomodulators (eg, advanced therapies) effectively achieve symptomatic remission in patients with inflammatory bowel disease (IBD). However, ~25%-50% of patients with IBD achieving symptomatic remission with an advanced therapy may have continued endoscopically/radiologically active bowel inflammation, and it is uncertain whether changing alternative advanced therapies in asymptomatic patients with IBD will reduce bowel inflammation and achieve durable deep remission.
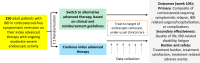
Methods and analysis: The QUality Outcomes Treating IBD to Target (QUOTIENT) study is an open-label, multicentre, pragmatic, randomised, controlled trial that aims to compare the efficacy and safety of switching to an alternative advanced therapy targeting endoscopic/radiological remission (treat-to-target) versus continuing the initial, or index, advanced therapy, in asymptomatic patients with IBD with moderate-to-severe endoscopic/radiological bowel inflammation. Enrolment is planned for ~250 participants in Canada/USA, randomised 1:1 to switching to alternative advanced therapy or continuing index advanced therapy, and then followed 104 weeks within routine clinical practice. Patient-reported outcomes measure efficacy and quality of life/treatment burden/safety. Primary endpoint is the time from randomisation to treatment failure.
Ethics and dissemination: The study is conducted in compliance with the protocol, ICH Good Clinical Practice, applicable regulatory requirements and appropriate review boards/independent ethics committees (approval numbers: Pro00077486; Pro00061437; STUDY00002062; 22-004171; i22-01269; IRB22-0890; IRB_00154397; 2000032384; SHIRB#2022.095-2; STUDY00007146; MMC#2024-18; REB#125290; 17784; Pro00142214; 20240660-01H), with documented written informed consent. Findings will be disseminated through peer-reviewed journals, scientific presentations, and publicly available Patient-Centered Outcomes Research Institute (PCORI) websites, including lay summaries. The Crohn's & Colitis Foundation Education, Support, and Advocacy Department, and our patient advocacy stakeholder, will develop educational and marketing resources to communicate findings to a broad audience (>250 000 patients/caregivers/healthcare professionals).
Trial registration number: NCT05230173.
Keywords: CROHN'S DISEASE; IMMUNOTHERAPY; INFLAMMATORY BOWEL DISEASE.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SS reports receiving research grants from Pfizer. DIF reports consulting/advisory board fees from Pfizer, Fresenius Kabi and Janssen. SAS reports being a consultant for Roche Information Systems. DH reports receiving research support from Johnson and Johnson and Pfizer; and consulting for AbbVie, Avalo, BMS, Corevitas, Eli Lilly, Johnson and Johnson, Pfizer, Prometheus, and Takeda. RAM has received consulting/advisory board fees from Pfizer, and received speaker’s fees from AbbVie, Takeda, Pfizer, Bristol Myers Squibb and Eli Lilly. DJL reports consultancy fees from Abbvie, Altrubio, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Johnson & Johnson, Palatin Technologies, Pfizer, Prime Therapeutics, Prometheus Laboratories, PSI, Takeda and Vedanta; has received speaker fees from AbbVie and Johnson & Johnson; and has received grants from AbbVie, Boehringer Ingelheim, Crohn’s & Colitis Foundation, Johnson & Johnson and Takeda. ADF has received consulting/advisory board fees from AbbVie; and reports receiving research support from Takeda. CH reports consulting/speaking with AbbVie, Janssen and Bristol Myers Squibb. JKJG reports receiving research support from Merck, AbbVie, Janssen, and Takeda. MCM reports speaking and consulting for Johnson and Johnson, AbbVie, Celltrion and Pfizer. DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Apex, Avalo Therapeutics, Bristol-Myers Squibb, Buhlmann Diagnostics Corp, Celgene, Connect BioPharma, Intouch Group, Iterative Health, Janssen Pharmaceuticals, Lilly, Pfizer, Samsung Neurologica, and Takeda. He serves on the Board of Trustees for the Crohn’s & Colitis Foundation and is on the Board of Directors for Cornerstones Health. JGH has served on an advisory board for Bristol Myers Squibb. TA has received consulting or speaker fees from AbbVie, Takeda, Janssen, Ferring and Fresenius Kabi. CM has received consulting fees from AbbVie, Alimentiv Inc., Amgen, AVIR Pharma Inc., Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Pendopharm, Pfizer, Prometheus Biosciences, Roche, Sanofi, Takeda, Tillotts Pharma; speaker's fees from AbbVie, Amgen, AVIR Pharma, Alimentiv Inc., Bristol Myers Squibb, Eli Lilly, Ferring, Fresenius Kabi, Janssen, Organon, Pendopharm, Pfizer, Sanofi, Takeda, Tillotts Pharma; royalties from Springer Publishing; and research support from AbbVie, Ferring, and Pfizer. FH has served on advisory boards or as speaker for Abbvie, Janssen, MSD, Takeda, Pfizer, Celltrion, Teva, Sandoz, Amgen and Pendopharm; and has received independent research funding from Janssen, Abbvie, Pfizer and Takeda. NN has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis and Ferring. TB has received speaker or consultant fees from AbbVie, Alimentiv Inc., BMS, CSF Vifor, Eli Lilly, Ferring, Fresenius Kabi, Gilead, Iterative scope, Janssen, Merck, Mirium, Pendopharm, Pentax, Pfizer, Roche, Sandoz, Sanofi, Takeda, Viatris. GR has received honoraria as a consultant and/or speaker for Abbvie, Celltrion, Fresenius Kabi, Ferring, Janssen, Merck, Organon, Pendopharm, Pfizer, Takeda, Sandoz and Viatris; he has received Research Grant Support from Abbvie, Ferring, Pfizer, and Crohn’s and Colitis Canada; and he has received educational grant support from Celltrion, Pfizer and Takeda. JDM reports potential conflicts with AbbVie, Amgen, Celltrion, Fresenius Kabbi, Pfizer, J and J, Takeda and Eli Lilly. RKC reports consulting and participation in advisory boards for AbbVie, Adiso, BMS, Janssen, Pfizer, Magellan Health, Option Care, Pharmacosmos, Pfizer, Samsung Bioepis, Sandoz and Sebela; speakers bureau for Pfizer; research grant from Janssen; and is a member of the Executive Committee for the IBD Education Group and Scientific Co-Director of the CorEvitas Registry. CAS reports Consultant/Advisory Board for Abbvie, BMS, Boomerang, Buhlmann, Janssen, Lilly, Napo Pharmaceuticals, Pfizer, Prometheus Biosciences, Prometheus Labs, Takeda and Trellus Health; speaker for CME activities for AbbVie, Janssen, Pfizer and Takeda; grant support from AbbVie, Janssen, Pfizer and Takeda; and licensing fees for Takeda. GYM has received consulting/advisory board fees from AbbVie, Arena Pharmaceuticals, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, Dieta Health, Eli Lilly, Enthera, Ferring, Fresenius Kabi, Genentech, Gilead, Iterative Health, Janssen, Medtronic, Oshi Health, OptionCare, Pfizer, Prometheus Labs, Rippling, Shield Therapeutics, Shionogi, Takeda, Techlab, Verantos, Viatris; and research support from Pfizer. SAW is an employee of the Crohn’s & Colitis Foundation. GZ has received consulting fees from Alimentiv Inc. VJ has received has received consulting/advisory board fees from AbbVie, Alimentiv Inc., Altruboi, Amgen, AnaptysBio, Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, Astra Zeneca, Avoro Capital, Bristol Myers Squibb, Calluna Pharma, Celltrion, Eli Lilly, Endpoint Health, Enthera, Exeliom Biosciences, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, Gilde Healthcare, GlaxoSmithKline, Genentech, Gilead, Innomar, JAMP, Janssen, Merck, Metacrine, Mylan, MRM Health, Mylan, Nxera Therapeutics, Organon, Pandion, Pendopharm, Pfizer, Protagonist, Reistone Biopharma, Roche, Roivant, Sandoz, Second Genome, Sorriso, Spyre Therapeutics, Synedgen, Takeda, TD Cowen, Tillotts, Topivert, Union Therapeutics, Ventyx, and Vividion; and received speaker’s fees from Abbvie, Ferring, Bristol Myers Squibb, Galapagos, Janssen, Pfizer, Takeda and Fresenius Kabi.
Figures
References
-
- Alatab S, Sepanlou SG, Ikuta K. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
