Ticagrelor Compared to Clopidogrel in Acute Coronary Syndromes trial (TC4): a Bayesian pragmatic cluster randomized controlled trial
- PMID: 40164463
- PMCID: PMC11957720
- DOI: 10.1503/cmaj.241862
Ticagrelor Compared to Clopidogrel in Acute Coronary Syndromes trial (TC4): a Bayesian pragmatic cluster randomized controlled trial
Abstract
Background: Dual antiplatelet therapy is the standard of care for acute coronary syndrome, but uncertainty exists regarding the optimal regimen for patients in North America. We sought to compare the effectiveness and safety of acetylsalicylic acid (ASA) and ticagrelor or clopidogrel in patients with acute coronary syndrome from a single tertiary academic centre in Montréal, Canada.
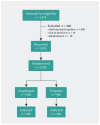
Methods: We conducted a pragmatic, open-label, time-clustered (bimonthly between October 2018 and March 2021), randomized controlled trial. The primary effectiveness end point was a composite of all-cause mortality, nonfatal myocardial infarction, or ischemic stroke. The primary safety end point was hospital admissions for bleeding. We ascertained 12-month outcomes from the Quebec universal electronic health databases. We designed and analyzed the study within a Bayesian paradigm to supplement existing knowledge. The primary analysis was a Bayesian logistic regression model with an informed focused prior from previously randomly assigned North American patients. Robustness was evaluated with vague and other prespecified informative priors, spanning reasonable pre-existing beliefs. We defined clinically important benefits and harms as risk reductions exceeding a 10% difference.
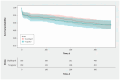
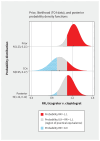
Results: We randomly assigned 1005 patients with acute coronary syndrome to ticagrelor (n = 450) or clopidogrel (n = 555). Major acute cardiovascular events occurred in 50 (11.1%) patients assigned to ticagrelor and 64 (11.5%) assigned to clopidogrel (relative risk [RR] 0.95, 95% credible interval 0.67-1.35, with a vague prior). The primary analysis with an informed focused prior resulted in probabilities of a clinically meaningful ticagrelor benefit (RR < 0.9), equivalence (0.9 ≤ RR ≤ 1.1) or harm (RR ≥ 1.1) of 2%, 41%, and 57%, respectively. For the safety end point, there was no consistent signal of benefit or harm with ticagrelor. Sensitivity analyses with a range of prior beliefs gave generally consistent results.
Interpretation: Whether we analyzed this trial with a vague or a range of reasonable informed priors, we found no strong evidence for the superiority of ticagrelor over clopidogrel in North American patients. Current guidelines favouring ticagrelor over clopidogrel might take this new evidence into future consideration.
Trial registration: Clinicaltrials.gov no. NCT04057300.
© 2025 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests:: None declared.
Figures
References
-
- Collet J-P, Thiele H, Barbato E, et al. .; ESC Scientific Document Group. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42: 1289–367. - PubMed
-
- Levine GN, Bates ER, Bittl JA, et al. . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol 2016;68:1082–115. - PubMed
-
- Mehta SR, Bainey KR, Cantor WJ, et al. . Members of the Secondary Panel. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol 2018;34:214–33. - PubMed
-
- Wallentin L, Becker RC, Budaj A, et al. . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. - PubMed
-
- Baker M. 1,500 scientists lift the lid on reproducibility. Nature 2016;533:452–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
