Generation and characterization of neutralizing antibodies against M1R and B6R proteins of monkeypox virus
- PMID: 40164599
- PMCID: PMC11958656
- DOI: 10.1038/s41467-025-58180-z
Generation and characterization of neutralizing antibodies against M1R and B6R proteins of monkeypox virus
Abstract
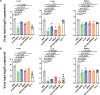
The global outbreak of monkeypox virus (MPXV), combined with the termination of smallpox vaccination and the lack of specific antiviral treatments, raises increasing concerns. The surface proteins M1R and B6R of MPXV are crucial for virus transmission and serve as key targets for vaccine development. In this study, a panel of human antibodies targeting M1R and B6R is isolated from a human antibody library using phage display technology. Among these antibodies, A138 against M1R and B026 against B6R show the most potent broad-spectrum neutralizing activities against MPXV and Vaccinia virus (VACV). When used in combination, A138 and B026 exhibit complementary neutralizing activity against both viruses in vitro. X-ray crystallography reveales that A138 binds to the loop regions of M1R, similar to the vulnerable epitope of 7D11 on VACV L1R. By contrast, A129 targets a more cryptic epitope, primarily comprising the β-strands of M1R. Moreover, prophylactic and therapeutic administration of A138 or B026 alone provides partial protection, while combining these two antibodies results in enhanced protection against VACV in male C57BL/6 mice. This study demonstrates of a dual-targeting strategy using two different components of the virion for the prevention and treatment of MPXV infection.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Patent applications have been filed that cover some of the antibodies presented here. GC, YQ, and WC are inventors. The other authors declare no competing interests.
Figures






References
-
- Arita, I., Jezek, Z., Khodakevich, L. & Ruti, K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am. J. Trop. Med Hyg.34, 781–789 (1985). - PubMed
-
- Agency. UHS. Guidance: monkeypox: background information, (2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

