PGC-1α mediates migrasome secretion accelerating macrophage-myofibroblast transition and contributing to sepsis-associated pulmonary fibrosis
- PMID: 40164683
- PMCID: PMC12046055
- DOI: 10.1038/s12276-025-01426-z
PGC-1α mediates migrasome secretion accelerating macrophage-myofibroblast transition and contributing to sepsis-associated pulmonary fibrosis
Abstract
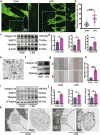
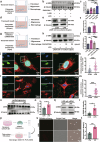
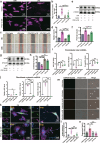
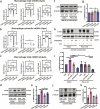
Sepsis-associated pulmonary fibrosis (SAPF) is a critical pathological stage in the progression of sepsis-induced acute respiratory distress syndrome. While the aggregation and activation of lung fibroblasts are central to the initiation of pulmonary fibrosis, the macrophage-myofibroblast transition (MMT) has recently been identified as a novel source of fibroblasts in this context. However, the mechanisms driving MMT remain inadequately understood. Given the emerging role of migrasomes (novel extracellular vesicles mediating intercellular communication), we investigated their involvement in pulmonary fibrosis. Here we utilized a lipopolysaccharide-induced SAPF mouse model and an in vitro co-culture system of fibroblasts and macrophages to observe the MMT process during SAPF. We found that lipopolysaccharide exposure suppresses PGC-1α expression in lung fibroblasts, resulting in mitochondrial dysfunction and the accumulation of cytosolic mitochondrial DNA (mtDNA). This dysfunction promotes the secretion of mtDNA-containing migrasomes, which, in turn, initiate the MMT process and contribute to fibrosis progression. Notably, the activation of PGC-1α mitigates mitochondrial dysfunction, reduces mtDNA-migrasome release, inhibits MMT and alleviates SAPF. In conclusion, our study identifies the suppression of PGC-1α in lung fibroblasts and the subsequent release of mtDNA migrasomes as a novel mechanism driving MMT in SAPF. These findings suggest that targeting the crosstalk between fibroblasts and immune cells mediated by migrasomes could represent a promising therapeutic strategy for SAPF.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Zhou, W. Q., Wang, P., Shao, Q. P. & Wang, J. Lipopolysaccharide promotes pulmonary fibrosis in acute respiratory distress syndrome (ARDS) via lincRNA-p21 induced inhibition of Thy-1 expression. Mol. Cell Biochem.419, 19–28 (2016). - PubMed
-
- Otoupalova, E., Smith, S., Cheng, G. & Thannickal, V. J. Oxidative stress in pulmonary fibrosis. Compr. Physiol.10, 509–547 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

