Senescence profiling of monoclonal gammopathies reveals paracrine senescence as a crucial defense against disease progression
- PMID: 40164720
- PMCID: PMC12055601
- DOI: 10.1038/s41375-025-02572-z
Senescence profiling of monoclonal gammopathies reveals paracrine senescence as a crucial defense against disease progression
Abstract
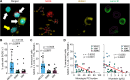
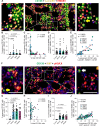
Multiple myeloma (MM) is a plasma cell (PC) malignancy that is preceded by monoclonal gammopathy of undetermined significance (MGUS) and/or smoldering multiple myeloma (SMM). MGUS and SMM PCs exhibit the same primary oncogenic abnormalities as MM but lack the end-organ damage that defines proliferative disease, suggesting that clonal PCs in these precursor conditions could exhibit senescence or senescence-like growth arrest. Herein we identified monoclonal gammopathy patient-derived PCs that exhibit senescence features and found that senescent PCs were significantly increased in MGUS patients compared to SMM or MM. Spatial analysis of senescent PCs in stable MGUS and SMM patient biopsies demonstrated the activation of local paracrine senescence in the bone marrow microenvironment. Stable MGUS and SMM patients also exhibited disease-specific senescence-associated secretory phenotype (SASP) signatures that significantly correlated with PC burden and clonal antibody. In contrast, progressing MGUS, SMM, and new MM patients lacked local paracrine senescence responses and robust activation of disease specific SASP signatures. Overall, these data suggest that failure to activate tumor-specific paracrine senescence responses is key to disease progression in monoclonal gammopathies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: TT and JLK have a financial interest related to this research, including patents and pending patents covering senolytic drugs and their uses held by Mayo Clinic. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies. Ethics approval and consent to participate: All methods described in this manuscript were performed in accordance with the relevant guidelines and regulations. The human study protocols reported in this manuscript were conducted in accordance with the Declaration of Helsinki. Patients who donated bone marrow aspiration samples and peripheral plasma provided written informed consent in accordance with the biobanking study protocol, which was approved by the Mayo Clinic Institutional Review Board under the number 521-93. Analyses of these samples was approved by the Mayo Clinic Institutional Review Board under the number 20-002263. Trephine iliac crest bone biopsies from MGUS, SMM, and NDMM patients were obtained from Danish pathological biobanks under approval from the Danish National Committee on Biomedical Research Ethics (S-20190110) and in compliance with the Declaration of Helsinki. All patients provided written informed consent. Bone biopsies from healthy individuals were included from a previous study approved by the Danish National Committee on Biomedical Research Ethics under protocol number 1-10-72-223-20, with informed consent obtained from all participants.
Figures




References
-
- American Cancer Society. Cancer Facts & Figures 2024. Atlanta: American Cancer Society. 2024.
-
- Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. - PMC - PubMed
-
- Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–90. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

