A cerebrospinal fluid synaptic protein biomarker for prediction of cognitive resilience versus decline in Alzheimer's disease
- PMID: 40164724
- PMCID: PMC12092275
- DOI: 10.1038/s41591-025-03565-2
A cerebrospinal fluid synaptic protein biomarker for prediction of cognitive resilience versus decline in Alzheimer's disease
Abstract
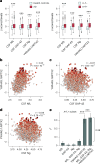
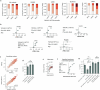
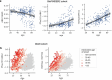
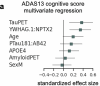
Rates of cognitive decline in Alzheimer's disease (AD) are extremely heterogeneous. Although biomarkers for amyloid-beta (Aβ) and tau proteins, the hallmark AD pathologies, have improved pathology-based diagnosis, they explain only 20-40% of the variance in AD-related cognitive impairment (CI). To discover novel biomarkers of CI in AD, we performed cerebrospinal fluid (CSF) proteomics on 3,397 individuals from six major prospective AD case-control cohorts. Synapse proteins emerged as the strongest correlates of CI, independent of Aβ and tau. Using machine learning, we derived the CSF YWHAG:NPTX2 synapse protein ratio, which explained 27% of the variance in CI beyond CSF pTau181:Aβ42, 11% beyond tau positron emission tomography, and 28% beyond CSF neurofilament, growth-associated protein 43 and neurogranin in Aβ+ and phosphorylated tau+ (A+T1+) individuals. CSF YWHAG:NPTX2 also increased with normal aging and 20 years before estimated symptom onset in carriers of autosomal dominant AD mutations. Regarding cognitive prognosis, CSF YWHAG:NPTX2 predicted conversion from A+T1+ cognitively normal to mild cognitive impairment (standard deviation increase hazard ratio = 3.0, P = 7.0 × 10-4) and A+T1+ mild cognitive impairment to dementia (standard deviation increase hazard ratio = 2.2, P = 8.2 × 10-16) over a 15-year follow-up, adjusting for CSF pTau181:Aβ42, CSF neurofilament, CSF neurogranin, CSF growth-associated protein 43, age, APOE4 and sex. We also developed a plasma proteomic signature of CI, which we evaluated in 13,401 samples, which partly recapitulated CSF YWHAG:NPTX2. Overall, our findings underscore CSF YWHAG:NPTX2 as a robust prognostic biomarker for cognitive resilience versus AD onset and progression, highlight the potential of plasma proteomics in replacing CSF measurement and further implicate synapse dysfunction as a core driver of AD dementia.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.W-C. and H.S.-H.O. are cofounders and scientific advisers of Teal Omics and have received equity stakes. C.C. has received research support from GSK and Eisai. C.C. is a member of the scientific advisory board of Circular Genomics and owns stocks. C.C. is a member of the scientific advisory board of ADmit. C.C. and M.A. have an invention disclosure for AT1 prediction models, including protein IDs, weights, cutoffs and algorithms. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he has received consultancy and speaker fees from ALZpath, BioArctic, Biogen, Bristol Myers Squibb, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. H.Z. has served at scientific advisory boards or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, Cognition Therapeutics, Denali, Eisai, Labcorp, Merry Life Biomedical, NervGen Pharma, Novo Nordisk, OptoCeutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by AlzeCure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk and Roche, and is a cofounder of Brain Biomarker Solutions in Gothenburg, which is a part of the GU Ventures Incubator Program (outside the submitted work). The other authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- U01 HL096812/HL/NHLBI NIH HHS/United States
- U01 HL096917/HL/NHLBI NIH HHS/United States
- P30AG066515/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P01 AG003991/AG/NIA NIH HHS/United States
- R21 AG058859/AG/NIA NIH HHS/United States
- 75N92022D00004/HL/NHLBI NIH HHS/United States
- RF1 AG074007/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- K99 AG088304/AG/NIA NIH HHS/United States
- R01 AG072255/AG/NIA NIH HHS/United States
- U01 HL096814/HL/NHLBI NIH HHS/United States
- 75N92022D00003/HL/NHLBI NIH HHS/United States
- 75N92022D00005/HL/NHLBI NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- U01 HL096899/HL/NHLBI NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
- P30 AG072975/AG/NIA NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG048076/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- U01 AG058922/AG/NIA NIH HHS/United States
- R01 AG044546/AG/NIA NIH HHS/United States
- 75N92022D00002/HL/NHLBI NIH HHS/United States
- AG072255/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- P30 AG066515/AG/NIA NIH HHS/United States
- RF1 AG053303/AG/NIA NIH HHS/United States
- U01 HL096902/HL/NHLBI NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- RF1 AG058501/AG/NIA NIH HHS/United States
- 75N92022D00001/HL/NHLBI NIH HHS/United States
- F32 AG079666/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

