Plasma MTBR-tau243 biomarker identifies tau tangle pathology in Alzheimer's disease
- PMID: 40164726
- PMCID: PMC12176612
- DOI: 10.1038/s41591-025-03617-7
Plasma MTBR-tau243 biomarker identifies tau tangle pathology in Alzheimer's disease
Abstract
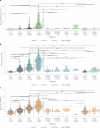
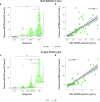
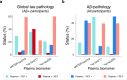
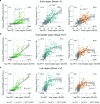
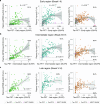
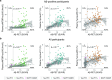
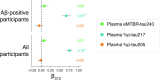
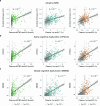
Insoluble tau aggregates within neurofibrillary tangles are a defining neuropathological feature of Alzheimer's disease (AD) and closely correlate with clinical symptoms. Although tau pathology can be assessed using tau positron emission tomography, a more accessible biomarker is needed for diagnosis, prognosis and tracking treatment effects. Here we present a new plasma tau species, the endogenously cleaved, microtubule-binding region containing residue 243 (eMTBR-tau243), which specifically reflects tau tangle pathology. Across the AD spectrum in three different cohorts (n = 108, 55 and 739), plasma eMTBR-tau243 levels were significantly elevated at the mild cognitive impairment stage and increased further in dementia. Plasma eMTBR-tau243 showed strong associations with tau positron emission tomography binding (β = 0.72, R2 = 0.56) and cognitive performance (β = 0.60, R2 = 0.40), outperforming other plasma tau (%p-tau217 and %p-tau205) biomarkers. These results suggest that plasma eMTBR-tau243 may be useful for estimating the tauopathy load in AD, thereby improving the diagnostic evaluation of AD in clinical practice and monitoring the efficacy of tau-targeted therapies in clinical trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.H. is an Eisai-sponsored voluntary research associate professor at Washington University and has received a salary from Eisai. Washington University, R.J.B. and D.M.H. have equity ownership interest in C2N Diagnostics. R.J.B. and D.M.H. receive income from C2N Diagnostics for serving on the scientific advisory board. K.H., N.R.B., C.S. and R.J.B. may receive income based on technology (Methods to detect MTBR tau isoforms and use thereof; PCT/US2020/046224) licensed by Washington University to C2N Diagnostics. K.H., H.J., D.M.H. and R.J.B. may receive income based on technology (Anti-tau MTBR antibodies and methods to detect endogenously cleaved fragments of tau and uses thereof; PCT/US2023/072738) licensed by Washington University to C2N Diagnostics. R.J.B. is an unpaid scientific advisory board member of Roche and Biogen, and receives research funding from Avid Radiopharmaceuticals, Janssen, Roche or Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb and Novartis. O.H. is an employee of Eli Lilly and Lund University and has previously acquired research support (for Lund University) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he has received consultancy or speaker fees from ALZpath, BioArctic, Biogen, Bristol Meyer Squibb, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. S.E.S. has served on scientific advisory boards or given lectures for Eisai, Eli Lilly and Novo Nordisk. D.M.H. is on the scientific advisory board of Genentech, Denali and Cajal Neurosciences and Switch, and consults for Asteroid and Roche. S.P. has acquired research support (for the institution) from ki elements or ADDF and Avid. In the past 2 years, he has received consultancy or speaker fees from BioArctic, Biogen, Eisai, Lilly, Novo Nordisk and Roche. The other authors declare no competing interests.
Figures





References
-
- van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med.10.1056/NEJMoa2212948 (2022). - PubMed
-
- Price, J. L. & Morris, J. C. Tangles and plaques in nondemented aging and ‘preclinical’ Alzheimer’s disease. Ann. Neurol.45, 358–368 (1999). - PubMed
-
- Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol.21, 726–734 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

