DELTA: a method for brain-wide measurement of synaptic protein turnover reveals localized plasticity during learning
- PMID: 40164741
- PMCID: PMC12081306
- DOI: 10.1038/s41593-025-01923-4
DELTA: a method for brain-wide measurement of synaptic protein turnover reveals localized plasticity during learning
Abstract
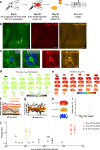
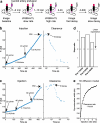
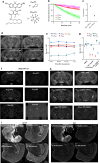
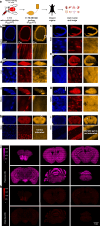
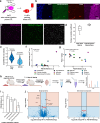
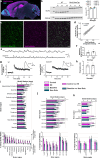
Synaptic plasticity alters neuronal connections in response to experience, which is thought to underlie learning and memory. However, the loci of learning-related synaptic plasticity, and the degree to which plasticity is localized or distributed, remain largely unknown. Here we describe a new method, DELTA, for mapping brain-wide changes in synaptic protein turnover with single-synapse resolution, based on Janelia Fluor dyes and HaloTag knock-in mice. During associative learning, the turnover of the ionotropic glutamate receptor subunit GluA2, an indicator of synaptic plasticity, was enhanced in several brain regions, most markedly hippocampal area CA1. More broadly distributed increases in the turnover of synaptic proteins were observed in response to environmental enrichment. In CA1, GluA2 stability was regulated in an input-specific manner, with more turnover in layers containing input from CA3 compared to entorhinal cortex. DELTA will facilitate exploration of the molecular and circuit basis of learning and memory and other forms of plasticity at scales ranging from single synapses to the entire brain.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
-
- Alberts, B. et al. Essential Cell Biology (W. W. Norton & Company, 2019).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

