Alzheimer mimicry: LATE and PART
- PMID: 40164861
- PMCID: PMC12568847
- DOI: 10.1007/s00702-025-02916-0
Alzheimer mimicry: LATE and PART
Abstract
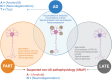
Alzheimer's disease (AD) is the main cause of dementia and accounts for 60% of dementia syndromes in people older than 75 years. The correct classification of AD and non-AD cases is mandatory to study disease mechanisms or new treatment possibilities. A typical clinical picture of AD consists of a progressive cognitive decline, with primary memory impairment. Structural, functional, and molecular brain imaging, along with CSF biomarkers of amyloid pathology, neurodegeneration, and the presence of a vulnerability-associated APOE genotype, support the diagnosis of AD. Use of biomarkers have led to the identification of individuals with mild cognitive impairment who are amyloid-negative addressing a conceptually separate clinical entity named suspected non-Alzheimer disease pathophysiology (SNAP). Clinical presentation and progression of SNAP can mimic AD which makes the final diagnosis and possible treatment uncertain in up to 30% of cases in clinical centers that are not using biomarkers. These non-AD pathologies are common with advancing age both in cognitively impaired and clinically normal elderly people and include Argyrophilic Grain Disease (ARG), Tangle Predominant Dementia and TDP-43 proteinopathy. The terms Primary age-related tauopathy (PART) and Limbic-dominant TDP-43 age-related encephalopathy (LATE) have been proposed as the most common and useful biological and emerging clinical construct to describe this phenomenon in > 80 years old individuals. Current evidence underlines the limitations of existing diagnostic tools, which remain inadequate for fully capturing the complexities of these conditions. Addressing these diagnostic ambiguities is crucial for assigning accurate diagnoses, reducing frequent misdiagnoses of AD, and implementing appropriate therapeutic strategies for elderly patients with mild cognitive impairment and dementia.
Keywords: Atypical Alzheimer; Dementia; Elderly; LATE; PART; SNAP.
© 2025. The Author(s).
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

