Does KarXT (xanomeline-trospium) represent a novel approach to schizophrenia management? A GRADE-assessed systematic review and meta-analysis of randomized controlled clinical trials
- PMID: 40165106
- PMCID: PMC11959844
- DOI: 10.1186/s12888-025-06696-5
Does KarXT (xanomeline-trospium) represent a novel approach to schizophrenia management? A GRADE-assessed systematic review and meta-analysis of randomized controlled clinical trials
Abstract
Background: Schizophrenia is a complex psychiatric disorder characterized by positive, negative, and cognitive symptoms. KarXT, a novel combination of xanomeline and trospium, offers potential therapeutic benefits for schizophrenia treatment by targeting muscarinic receptors and avoiding dopamine receptor blockade. We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of KarXT.
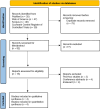
Methods: PubMed, Scopus, Web of Science, and Cochrane databases were systematically searched for relevant randomized controlled trials (RCTs) up to October 2024. Studies involving adult patients with schizophrenia treated with KarXT were included. Furthermore, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was used to assess evidence quality, and the risk of bias was evaluated using the Cochrane Risk of Bias 2.0 tool.
Results: Four studies with 690 participants were included. KarXT significantly reduced Positive and Negative Syndrome Scale (PANSS) total scores compared to placebo (mean difference (MD): -13.77, 95% confidence interval (CI) [-22.33 to -5.20], P-value = 0.002), with significant improvements in positive and negative subscale scores. It significantly increased the incidence of achieving ≥ 30% PANSS score reduction (risk ratio: 2.15, 95% CI [1.64 to 2.84], P < 0.00001). Moreover, KarXT demonstrated a favorable safety profile, with side effects such as nausea and constipation being mild and transient. Notably, it was not significantly associated with weight gain or extrapyramidal symptoms, which are common with traditional antipsychotics.
Conclusions: KarXT's distinct mechanism and tolerability highlight its potential to address unmet needs in schizophrenia treatment. Future studies should explore its long-term efficacy, delayed adverse effects, and comparative effectiveness against existing therapies.
Clinical trial number: Not applicable.
Keywords: KarXT; Meta-analysis; Schizophrenia; Systematic review; Trospium; Xanomeline; Xanomeline-trospium.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Human ethics and consent to participate statement: Our manuscript was not applied to human beings and thus requires no ethical approval. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Abidi S. Psychosis in children and youth: focus on early-onset schizophrenia. Pediatr Rev. 2013;34:296–305. quiz 305. - PubMed
-
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. - PubMed
-
- Javitt DC. Balancing therapeutic safety and efficacy to improve clinical and economic outcomes in schizophrenia: a clinical overview. Am J Manag Care. 2014;20(8 Suppl):S160–5. - PubMed
-
- van Os J, Kapur S, Schizophrenia. Lancet. 2009;374:635–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

