Evaluating cloudcare, a population health management system, in persons with type 1 diabetes: an observational study
- PMID: 40165180
- PMCID: PMC11956218
- DOI: 10.1186/s12902-025-01905-4
Evaluating cloudcare, a population health management system, in persons with type 1 diabetes: an observational study
Abstract
Background: Innovations in diabetes technology have consistently improved outcomes of persons with type1 diabetes (PWDs). However, the volumes of data that these technologies yield require different workflows to alleviate healthcare professionals' (HCPs) workload and prevent losing relevant data in between visits for interpretation and treatment adaptations. CloudCare is a population health management tool that continuously oversees data from groups of individual PWDs, based on remote monitoring, screening and triaging of individual PWDs. This study assesses the effect of CloudCare on treatment satisfaction of PWDs, HCPs' workload and glycemic control of PWDs.
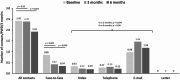
Methods: We evaluated the 6-month follow-up outcomes as part of an ongoing prospective cohort study analyzing the effect of CloudCare. Adult PWDs diagnosed > 6 months before inclusion were enrolled. The primary outcome was the change in PWD treatment satisfaction (DTSQc). Secondary outcomes included the number and type of contacts between HCPs and PWDs, diabetes-related distress (PAID-5), and glycemic control.
Results: In September 2024, 175 participants had baseline data available, with a median age of 29.9 years and a median diabetes duration of 17 years. Differences between baseline and 6 months could be calculated for 119 participants. After 6 months follow-up, the median increase in PWDs' treatment satisfaction (DTSQc) was + 6.0 (IQR 2-11; p < 0.001). The number of face-to-face contacts per PWD per 3 months decreased from 0.85 at baseline to 0.34 (p < 0.001) at 6 months. Diabetes-related distress was significantly decreased at 3 months (p < 0.001) and at 6 months (p = 0.034), compared with baseline. Glucometrics did not significantly change, with a TIR of 79% at baseline and 78% after 6 months (p = 0.39), and a mean glucose management indicator (GMI) of 50 mmol/mol (6.7%) at all timepoints.
Conclusions: In adult PWDs with good glycemic control, CloudCare decreases workload for HCPs, while increasing PWDs' treatment satisfaction and maintaining excellent glycemic control during 6 months, showing this concept can be applied in modern diabetes care with high density data availability.
Trial registration: Clinicaltrials.gov identifier: NCT05431140; registration date 21-6-2023.
Keywords: Care pathways; Population health management; Remote monitoring; Type 1 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Medical Research Ethics Committee of Erasmus Medical Centre (EMC), Rotterdam, The Netherlands, declared that since participants were not subjected to any actions or restrictions and followed in regular care, this study was exempt from further approval procedures (registration number MEC-2019-0790). Consent for publication: Not applicable. Competing interests: Diabeter is a pediatric and adult diabetes care and research clinic, owned by Medtronic, but with independent clinical care and prescription. CloudCare is a Medtronic product: the concept of CloudCare is developed fully independently by the medical staff of Diabeter. Medtronic has supported its development with IT and organizational manpower support. There has been no financial support for this work that could have influenced its outcome. SL is employee of Diabeter Nederland and of Medtronic Trading NL B.V. Eindhoven, the Netherlands.
Figures


References
-
- Dovc K, Battelino T. Evolution of diabetes technology. Endocrinol Metab Clin North Am. 2020;49(1):1–18. - PubMed
-
- Prigge R, McKnight JA, Wild SH, Haynes A, Jones TW, Davis EA, Rami-Merhar B, Fritsch M, Prchla C, Lavens A, et al. International comparison of glycaemic control in people with type 1 diabetes: an update and extension. Diabet Med. 2022;39(5):e14766. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

