User journey method: a case study for improving digital intervention use measurement
- PMID: 40165237
- PMCID: PMC11959768
- DOI: 10.1186/s12913-025-12641-9
User journey method: a case study for improving digital intervention use measurement
Abstract
Background: Many digital mental health interventions meet low levels of use. However, current use measurement methods do not necessarily help identify which intervention elements are associated with dropout, despite this information potentially facilitating iterative intervention development. Here, we suggest improving the comprehensiveness of intervention use measurement with the user journey method, which evaluates every intervention element to identify intervention-specific use barriers.
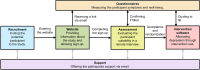
Methods: We applied user journey method in a clinical trial that investigated the efficacy of a novel game-based intervention, Meliora, for adult Major Depressive Disorder. We modelled the intervention for its four technological (Recruitment, Website, Questionnaires, Intervention Software) and two interpersonal elements (Assessment, Support). We then applied the user journey method to measure how many users proceeded from one element to the next combining social media analytics, website use data, signup data, clinical subject coordinator interview data, symptom questionnaire data, and behavioral intervention use data. These measurements were complemented with the qualitative analysis of the study discovery sources and email support contacts.
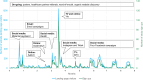
Results: Recruitment: The intervention recruitment reached at least 145,000 Finns, with social media, word-of-mouth, and news and web sources being the most effective recruitment channels. Website: The study website received 16,243 visitors, which led to 1,007 sign-ups.
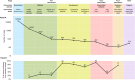
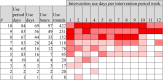
Assessment: 895 participants were assessed and 735 were accepted. Intervention Software: 498 participants were assigned to the active intervention or comparator, of whom 457 used them at least once: on average, for 17.3 h (SD = 20.4 h) on 19.7 days (SD = 20.7 d) over a period of 38.9 days (SD = 31.2 d). The 28 intervention levels were associated with an average dropout rate of 2.6%, with two sections exhibiting an increase against this baseline. 150 participants met the minimum adherence goal of 24 h use. Questionnaires: 116 participants completed the post-intervention questionnaire.
Support: 313 signed-up participants contacted the researchers via email.
Conclusion: The user journey method allowed for the comprehensive evaluation of the six intervention elements, and enabled identifying use barriers expediting iterative intervention development and implementation.
Trial registration: ClinicalTrials.gov, NCT05426265. Registered 28 June 2022, https://clinicaltrials.gov/ct2/show/NCT05426265 .
Keywords: Acceptability; Depression; Digital interventions; Engagement; Evaluation methods; Implementation; Mental health; Mixed methods; Use data; User-centered design.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The randomized controlled trial (RCT) received positive appraisals from the Helsinki University Hospital research ethics committee (HUS/3042/2021) and the Finnish Medicines Agency Fimea (FIMEA/2022/002976), and was preregistered on ClinicalTrials.gov [54]. Written informed consent was obtained from all participants at sign-up. Consent for publication: Not needed. Competing interests: LL and JMP are co-founders of Soihtu DTx Ltd., which develops game-based digital mental health interventions.
Figures

References
-
- Folker AP, Mathiasen K, Lauridsen SM, Stenderup E, Dozeman E, Folker MP. Implementing internet-delivered cognitive behavior therapy for common mental health disorders: A comparative case study of implementation challenges perceived by therapists and managers in five European internet services. Internet Interv. 2018;11:60–70. - PMC - PubMed
-
- Waller R, Gilbody S. Barriers to the uptake of computerized cognitive behavioural therapy: a systematic review of the quantitative and qualitative evidence. Psychol Med. 2009;39(5):705–12. - PubMed
-
- Lipschitz JM, Van Boxtel R, Torous J, Firth J, Lebovitz JG, Burdick KE, et al. Digital mental health interventions for depression: scoping review of user engagement. J Med Internet Res. 2022;24(10):e39204. Available from: https://www.jmir.org/2022/10/e39204. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

