A ROR1 targeted bispecific T cell engager shows high potency in the pre-clinical model of triple negative breast cancer
- PMID: 40165319
- PMCID: PMC11956192
- DOI: 10.1186/s13058-025-02005-w
A ROR1 targeted bispecific T cell engager shows high potency in the pre-clinical model of triple negative breast cancer
Abstract
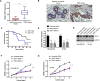
Background: Triple negative breast cancer (TNBC) is an aggressive breast cancer subtype characterized with poor prognosis and high metastatic potential. Although traditional chemotherapy, radiation, and surgical resection remain the standard treatment options for TNBC, bispecific antibody-based immunotherapy is emerging as new strategy in TNBC treatment. Here, we found that the receptor tyrosine kinase-like Orphan Receptor 1 (ROR1) was highly expressed in TNBC but minimally expressed in normal tissue. A bispecific ROR1-targeted CD3 T cell engager (TCE) was designed in IgG-based format with extended half-life.
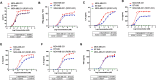
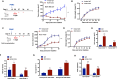
Method: The expression of ROR1 in TNBC was detected by RT-qPCR and immunohistology analysis. The killing of ROR1/CD3 antibody on TNBC cells was determined by the in vitro cytotoxicity assay and in vivo PBMC reconstituted mouse model. The activation of ROR1/CD3 on T cells was analyzed by the flow cytometry and ELISA assay. Pharmacokinetics study of ROR1/CD3 was performed in mouse.
Results: The ROR1/CD3 TCE triggered T cell activation and proliferation, which showed potent and specific killing to TNBC cells in ROR1-depedent manner. In vivo mouse model indicated that ROR1/CD3 TCE redirected the cytotoxic activity of T cells to lyse TNBC cells and induced significant tumor regression. Additionally, the ROR1/CD3 bispecific antibody exhibited an extended half-life in mouse, which may enable intermittent administration in clinic.
Conclusions: Collectively, these results demonstrated that ROR1/CD3 TCE has a promising efficacy profile in preclinical studies, which suggested it as a possible option for the treatment of ROR1-expressing TNBC.
Keywords: Immunotherapy; ROR1; T cell engager; TNBC.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Potent and selective antitumor activity of a T cell-engaging bispecific antibody targeting a membrane-proximal epitope of ROR1.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5467-E5476. doi: 10.1073/pnas.1719905115. Epub 2018 May 29. Proc Natl Acad Sci U S A. 2018. PMID: 29844189 Free PMC article.
-
Inducible localized delivery of an anti-PD-1 scFv enhances anti-tumor activity of ROR1 CAR-T cells in TNBC.Breast Cancer Res. 2022 Jun 3;24(1):39. doi: 10.1186/s13058-022-01531-1. Breast Cancer Res. 2022. PMID: 35659040 Free PMC article.
-
A prodrug nanodevice co-delivering docetaxel and ROR1 siRNA for enhanced triple negative breast cancer therapy.Acta Biomater. 2025 Jan 24;193:498-513. doi: 10.1016/j.actbio.2024.12.055. Epub 2024 Dec 25. Acta Biomater. 2025. PMID: 39730101
-
Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3ß activity.PLoS One. 2019 May 31;14(5):e0217789. doi: 10.1371/journal.pone.0217789. eCollection 2019. PLoS One. 2019. PMID: 31150511 Free PMC article.
-
Bispecific Antibodies for Triple Negative Breast Cancer.Trends Cancer. 2021 Feb;7(2):162-173. doi: 10.1016/j.trecan.2020.09.004. Epub 2020 Oct 8. Trends Cancer. 2021. PMID: 33041246 Review.
References
-
- Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33(6):637–45. - PubMed
-
- Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control. 2010;17(3):173–6. - PubMed
-
- da Silva JL, Cardoso Nunes NC, Izetti P, de Mesquita GG, de Melo AC. Triple negative breast cancer: A thorough review of biomarkers. Crit Rev Oncol Hematol. 2020;145:102855. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

